1. Introduction
Dyes are widely used as probes for the detection of different analytes in solution, such as metal ions, anions, small molecules, and biological macromolecules, [
1] as well as in solar cell [
2] or in catalysis for photoinduced water oxidation [
3]. In particular, among these fluorescent molecules have always attracted the interest of scientists, given their possible use in multidisciplinary areas. In the wide list of fluorescent organic compounds, the difluoro-boroindacene derivatives or BODIPY, have been recognized as the most versatile and their popularity has incredibly grown in recent decades [
4].
To date, thousands of new patents and articles on these derivatives are published with applications in all areas of scientific research. BODIPY have spectral characteristics that can be easily modulated with respect to well-known chromophores, such as porphyrins, fluoresceins, or rhodamines. Substituents on the BODIPY structure (on pyrrole carbons, on 8 or
meso position and on the boron atom) can modify the photophysical properties of the dye [
5,
6]. Introducing suitable groups into the right positions of the BODIPY core is essential for the expected chromophore and fluorophore setup. Indeed, the meso position is particularly sensitive to the substituent effect as already reported [
7].
The fluorescence of meso alkyl-BODIPY solutions is usually green (absorption-emission maxima around 500–520 nm) but in the presence of substituents conjugated to the central core, both the absorption and emission spectra show a strong bathochromic shift, with possible maximum emissions exceeding 750 nm for some derivatives. BODIPY also has high molar extinction coefficients (ε > 50,000 cm−1·M−1), high fluorescence quantum yield values (which often approaches 1.0, even in water), spectra relatively insensitive to the polarity and pH of the solvent, narrow bandwidth, fluorescence red shift at high concentration values (a property that can be used to detect regions of different densities), relatively long- lived excited state (typically 5 ns or more), useful for testing based on fluorescence polarization.
2. Results and Discussion
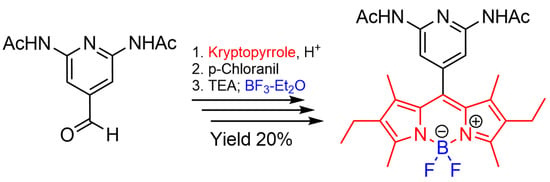
In this communication, the synthesis of a BODIPY from 3-ethyl-2,4-dimethyl-1
H-pyrrole (kryptopyrrole) and 2,6-diacetamido-4-formylpyridin is reported. The procedure involves the sequential one-pot addition of the various reagents. Kryptopyrrole (2.2 eq), aldehyde 1 (1 eq) and few drops of trifluoroacetic acid (TFA) in anhydrous dichloromethane (DCM) are added and the mixture was stirred for 4 days at room temperature in argon atmosphere. Then, a solution of p-chloranil (1 eq) in DCM is added to the mixture causing the solution to turn dark red. Triethylamine (TEA) (9 eq) is added and after 30 min an ethereal solution of BF
3 (6 eq) is added and stirred for 24 h. After extraction and purification by chromatographic column, 5-(2,6-diacetamidopyridin-4-yl)-kryptoBODIPY or DAAP-KBD
2 is separated with a yield of 20% as a dark red solid (
Scheme 1).
It is widely recognized that the BODIPY synthetic mechanism passes through the formation of two intermediates, dipyrromethane derivative and dipyrromethene and finally the complexation of BF
3 [
8].
Scheme 2 shows the plausible mechanism of the synthesis of DAAP-KBD
2, in line with what is already known.
To confirm this sequence, dipyrromethene
4 was isolated as a dark red byproduct, in the purification step of the reaction mixture. The synthesis of DAAP-KBD
2 from pure dipyrromethene
4 was also evaluated. Considering not high yields of
4 (about 30%) and yield conversion to BODIPY
2 about 60%, besides laborious purification procedure of
4, it can be said that one-pot procedure is more convenient for the synthesis of
2. The conversion to BODIPY from dipyrromethene is not quantitative probably due to the presence of a nucleophilic pyridine nitrogen which could negatively influence the boron complexation the dipyrromethene moiety [
9].
Some spectroscopic data of DAAP-KBD
2 and
4 are reported in
Table 1. DAAP-KBD
2 shows in DCM absorption and emission bands centered at 530 and 548 nm, respectively (Figure ESI). The narrow spectroscopic features with low Stokes Shift value (620 cm
−1) and mirror-symmetrical fluorescence band with respect to absorption band is typical of BODIPY dyes. The chromophore obeys to Lambert Beer’s law up to millimolar concentration with a molar extinction coefficient of 32,850 M
−1·cm
−1 in DCM, calculated in correspondence to absorption maximum band. These data indicate that no aggregates are formed under our experimental conditions which is also confirmed by the measurement of the fluorescence lifetime of the excited state, whose value of around 5 ns is consistent with the presence of monomer in solution. The corresponding DAAP-dipyrromethene
4 shows a better solubility in methanol than in DCM, therefore the spectroscopic data are reported in alcoholic solution. The compound shows a broad absorption band at 528 nm and a weak emission at around 544 nm (Figure ESI), this latter does not allow to make fluorescence lifetime measurement.
The design of DAAP-KBD
2 is in continuation to our recent studies [
10]. The ability to form supramolecular dimers of a similar DAAP-BODIPY with complementary uracil BODIPY [
11] was investigated. In
2 the modification of the substituents on the chromophoric core increases the electron density towards the center which alters the electron distribution capacity. This flexible approach could open the way for accessing to a plethora of programmable dyes and enables a systematic study of the principal factors affecting the dynamics of the energy migration and possibly driving energy transfer mechanisms, as previously reported [
10].
3. Materials and Methods
The solvents and chemicals were procured from commercial chemical suppliers and used without further purification. Reactions were monitored by thin-layer chromatography (TLC) on silica gel 60 F
254 aluminum plates. For purification, column chromatography was performed using 100−200 mesh silica gel. All nuclear magnetic resonance spectra were recorded with Varian 500 instruments using tetramethylsilane (TMS) as internal standard. Chemical shifts (δ) are given from TMS (δ = 0.00) in parts per million (ppm) with reference to the residual nuclei of the deuterated solvent used (CDCl
3:
1H-NMR δ = 7.26 ppm (s);
13C-NMR δ = 77.0 ppm). All
13C-NMR spectra were obtained with complete proton decoupling. Melting points were determined using a BÜCHI B-545 apparatus. The mass analyzes were performed using a high-resolution mass spectrometer Xevo G2-S (Waters) with Q-TOF analyzer. The UV-Vis absorption spectra were recorded using the Hewlett-Packard mod. 8453 diode array. The spectra of static fluorescence emission were recorded with Horiba Jobin Yvon-Spex Fluoromax 4 spectrofluorimeter and, for time resolved fluorescence measurements, time-correlated single-photon counting technique with a 390 nm NanoLED as excitation source has been used. The emission quantum yield (Φ) was measured relative to Rhodamine B in ethanol (Φ = 0.65) [
12] and corrected for refractive index of the solvent. The quantum yield of fluorescence (Φ) was calculated according to the following equation:
where
I is the fluorescence intensity at the maximum of the emission band,
Abs is the absorbance at the excitation wavelength and
n is the refractive index. The subscript
R is referred to the reference.
One-pot synthetic procedure of DAAP-KBD 2: 2,6-diacetamido-4-formylpyridine 1 (100 mg, 0.45 mmol), kryptopyrrole (2.2 eq) and a catalytic quantity of TFA, are dissolved in anhydrous DCM and the reaction was kept under stirring for 4 days at room temperature in inert atmosphere (Ar). A solution of p-chloranil (112 mg, 1 eq) in anhydrous DCM was added to the reaction mixture. After 1 h the color of the mixture becomes dark, as proof of oxidation and TEA (500 µL, 9 eq) was added. A solution of boron trifluoride (0.25 mL, 37% in diethyl ether, 6 eq) in anhydrous DCM (5 mL) was injected dropwise over a period of approximately 1 h and stirred for 24 h. The reaction mixture was subjected to two extractions with saturated solutions of NaHCO3 and brine, respectively. The organic phase was dehydrated with modest quantities of anhydrous Na2SO4, filtered, and evaporated under reduced pressure. Separation in chromatographic column (SiO2, 40% AcOEt/DCM) provided the 2 as dark red solid (45 mg, 20%); mp 208–215 °C.
2: 1H-NMR (500 MHz, CDCl3) δ 7.91 (s, 2H), 7.80 (b, 2H), 2.52 (s, 6H), 2.29 (q, 4H), 2.22 (s, 6H), 1.25 (s, 6H), 0.98 (t, 6H). 13C-NMR (125 MHz, CDCl3) δ 168.7, 165.4, 150.4, 148.5, 142.9, 138.6, 135.4, 129.2, 109.4, 31.2, 30.0, 25.1, 17.5, 15.1, 14.9, 12.0. 19F-NMR (376 MHz, CDCl3) δ -146.2 (q, 2F). 11B-NMR (160 MHz, CDCl3) δ 1.2 (t, 2JB-F = 32 Hz). HRMS (MALDI-TOF) m/z: [M + H]+ Calcd for C26H33BF2N5O2+ 496.269; Found 496.281.
DAAP-dipyrromethene 4: 2,6-diacetamido-4-formylpyridine 1 (100 mg, 0.45 mmol), kryptopyrrole (2.2 eq) and a catalytic quantity of TFA, are dissolved in anhydrous DCM and the reaction was kept under stirring for 4 days at room temperature in inert atmosphere (Ar). A solution of p-chloranil (112 mg, 1 eq) in anhydrous DCM was added to the reaction mixture. After 1 h the solvent was removed by rotavaporation and separation was carried out by chromatography column (SiO2, 10% MeOH/DCM) and 4 (60 mg, 30%), as dark red solid was isolated, showing good solubility in water and in methanol; mp 241–245 °C.
4: 1H-NMR (500 MHz, CD3OD) δ 7.81 (s, 2H, NH), 2.57 (q, J = 8.5 Hz, 4 H, CH2), 2.47 (s, 6H, CH3), 2.32 (s, 6H, CH3), 1.78 (s, 6H, CH3), 1.18 (t, J = 8.6 Hz, 6H, CH3). 13C-NMR (125 MHz, DMSO-d6) δ 170.4, 159.5, 153.9, 151.6, 147.3, 143.2, 130.3, 121.7, 117.1, 24.6, 17.5, 14.6, 11.3.
HRMS (MicrOTOF) m/z: [M + H]+ Calcd for C26H34N5O2+ 448,271; Found 448.244.
DAAP-KBD 2: 4 (60 mg, 0.134 mmol) was dissolved in anhydrous DCM (15 mL) in an inert atmosphere (Ar) and TEA (450 µL, 9 eq) was added. A solution of boron trifluoride (0.22 mL, 37% in diethyl ether, 6 eq) in anhydrous DCM (5 mL) was injected dropwise over a period of approximately 1 h. The work-up and purification procedures are identical to those described for the one-pot procedure. A dark red solid 2 was isolated (40 mg, 60%).