Potassium (3-Methyl-2-oxido-1,2,5-oxadiazol-4-yl)dinitromethanide
Abstract
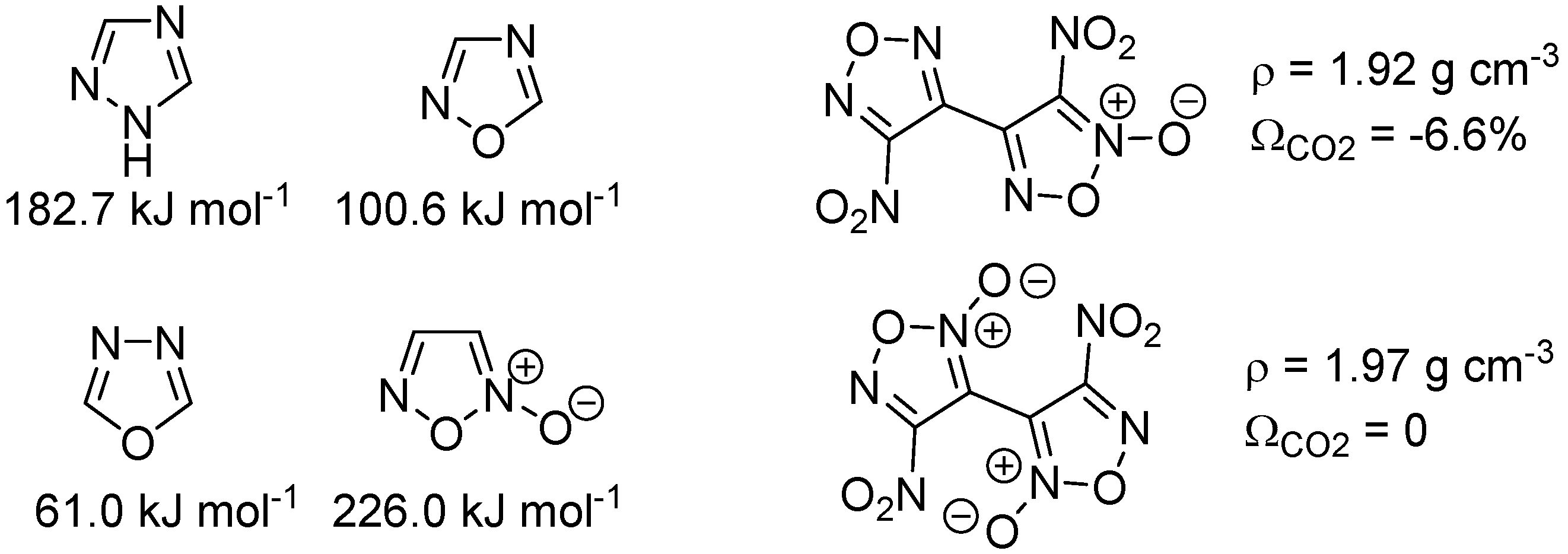
:1. Introduction
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Sullivan, O.T.; Zdilla, M.J. Properties and Promise of Catenated Nitrogen Systems as High-Energy—Density Materials. Chem. Rev. 2020, 120, 5682–5744. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, C. Review of the Molecular and Crystal Correlations on Sensitivities of Energetic Materials. J. Hazard. Mater. 2020, 398, 122910. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, J.J.; Johnson, E.C. A Short Review of Nitric Esters and Their Role in Energetic Materials. ACS Omega 2021, 6, 11813–11821. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Shem-Tov, D.; Wang, S.; Zhang, L.; Flaxer, E.; Zhang, S.; Stierstorfer, J.; Wang, K.; Yan, Q.-L.; Dobrovetsky, R.; et al. Hydride- and boron-free solid hypergolic H2O2-ignitophores. Chem. Eng. J. 2021, 426, 131806. [Google Scholar] [CrossRef]
- Luo, S.-N.; Gozin, M. Energetic Materials: Novel Syntheses and Diagnostics. Engineering 2020, 6, 974–975. [Google Scholar] [CrossRef]
- Larin, A.A.; Fershtat, L.L. High-Energy Hydroxytetrazoles: Design, Synthesis and Performance. Energ. Mater. Front. 2021, 2, 3–13. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M.; Khimeche, K. Tetrazole-Functionalized Microcrystalline Cellulose: A Promising Biopolymer for Advanced Energetic Materials. Chem. Eng. J. 2020, 400, 125960. [Google Scholar] [CrossRef]
- Snyder, C.J.; Imler, G.H.; Chavez, D.E.; Parrish, D.A. Synergetic Explosive Performance through Cocrystallization. Cryst. Growth Des. 2021, 21, 1401–1405. [Google Scholar] [CrossRef]
- Kuchurov, I.V.; Zharkov, M.N.; Fershtat, L.L.; Makhova, N.N.; Zlotin, S.G. Prospective Symbiosis of Green Chemistry and Energetic Materials. ChemSusChem 2017, 10, 3914–3946. [Google Scholar] [CrossRef]
- Simpson, R.L.; Urtiew, P.A.; Ornellas, D.L.; Moody, G.L.; Scribner, K.J.; Hoffman, D.M. CL-20 performance exceeds that of HMX and its sensitivity is moderate. Propellants Explos. Pyrotech. 1997, 22, 249–255. [Google Scholar] [CrossRef]
- Fedyanin, I.V.; Lyssenko, K.A.; Fershtat, L.L.; Muravyev, N.V.; Makhova, N.N. Crystal Solvates of Energetic 2,4,6,8,10,12-Hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane Molecule with [bmim]—Based Ionic Liquids. Cryst. Growth Des. 2019, 19, 3660–3669. [Google Scholar] [CrossRef]
- Zhang, M.; Eaton, P.E.; Gilardi, R. Hepta-and octanitrocubanes. Angew. Chem. Int. Ed. 2000, 39, 401–404. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J.; Wang, B.; Qiu, L.; Xu, R.; Sheremetev, A.B. Recent Synthetic Efforts towards High Energy Density Materials: How to Design High-Performance Energetic Structures? FirePhysChem 2021, in press. [Google Scholar] [CrossRef]
- Wozniak, D.R.; Salfer, B.; Zeller, M.; Byrd, E.F.C.; Piercey, D.G. Tailoring Energetic Sensitivity and Classification through Regioisomerism. Org. Lett. 2020, 22, 9114–9117. [Google Scholar] [CrossRef] [PubMed]
- Yount, J.R.; Zeller, M.; Byrd, E.F.C.; Piercey, D.G. 4,4′,5,5′-Tetraamino-3,3′-azo-bis-1,2,4-triazole and the electrosynthesis of high-performing insensitive energetic materials. J. Mater. Chem. A 2020, 8, 19337–19347. [Google Scholar] [CrossRef]
- Wurzenberger, M.H.; Gruhne, M.S.; Lommel, M.; Braun, V.; Szimhardt, N.; Stierstorfer, J. Taming the Dragon: Complexation of Silver Fulminate with Nitrogen-Rich Azole Ligands. Inorg. Chem. 2020, 59, 17875–17879. [Google Scholar] [CrossRef] [PubMed]
- Voronin, A.A.; Fedyanin, I.V.; Churakov, A.M.; Pivkina, A.N.; Muravyev, N.V.; Strelenko, Y.A.; Klenov, M.S.; Lempert, D.B.; Tartakovsky, V.A. 4H-[1,2,3]Triazolo[4,5-c][1,2,5]oxadiazole 5-oxide and Its Salts: Promising Multipurpose Energetic Materials. ACS Appl. Energy Mater. 2020, 3, 9401–9407. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Makhova, N.N. 1,2,5-Oxadiazole-Based High-Energy-Density Materials: Synthesis and Performance. ChemPlusChem 2020, 85, 13–42. [Google Scholar] [CrossRef] [Green Version]
- Bystrov, D.M.; Fershtat, L.L.; Makhova, N.N. Synthesis and reactivity of aminofuroxans. Chem. Heterocycl. Compd. 2019, 55, 1143–1164. [Google Scholar] [CrossRef]
- Larin, A.A.; Bystrov, D.M.; Fershtat, L.L.; Konnov, A.A.; Makhova, N.N.; Monogarov, K.A.; Meerov, D.B.; Melnikov, I.N.; Pivkina, A.N.; Kiselev, V.G.; et al. Nitro-, Cyano-, and Methylfuroxans, and Their Bis-Derivatives: From Green Primary to Melt-Cast Explosives. Molecules 2020, 25, 5836. [Google Scholar] [CrossRef]
- Larin, A.A.; Shaferov, A.V.; Epishina, M.A.; Melnikov, I.N.; Muravyev, N.V.; Ananyev, I.V.; Fershtat, L.L.; Makhova, N.N. Pushing the Energy-Sensitivity Balance with High-Performance Bifuroxans. ACS Appl. Energy Mater. 2020, 3, 7764–7771. [Google Scholar] [CrossRef]
- Larin, A.A.; Fershtat, L.L.; Makhova, N.N. Tandem Reactions of Thermolysis and [3+2] Cycloaddition in the Synthesis of 3-Hetaryl-4-Nitrofuroxans from 4-Nitrofuroxannitrolic Acid. Chem. Heterocycl. Compd. 2020, 56, 607–610. [Google Scholar] [CrossRef]
- Larin, A.A.; Muravyev, N.V.; Pivkina, A.N.; Suponitsky, K.Y.; Ananyev, I.V.; Khakimov, D.V.; Fershtat, L.L.; Makhova, N.N. Assembly of Tetrazolylfuroxan Organic Salts: Multipurpose Green Energetic Materials with High Enthalpies of Formation and Excellent Detonation Performance. Chem. Eur. J. 2019, 25, 4225–4233. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Shreeve, J.M. Potassium 4,5-Bis (dinitromethyl) furoxanate: A Green Primary Explosive with a Positive Oxygen Balance. Angew. Chem. Int. Ed. 2016, 55, 772–775. [Google Scholar] [CrossRef]
- Liu, Y.; He, C.; Tang, Y.; Imler, G.H.; Parrish, D.A.; Shreeve, J.M. Asymmetric nitrogen-rich energetic materials resulting from the combination of tetrazolyl, dinitromethyl and (1,2,4-oxadiazol-5-yl)nitroamino groups with furoxan. Dalton Trans. 2018, 47, 16558–16566. [Google Scholar] [CrossRef] [PubMed]
- Fershtat, L.L.; Larin, A.A.; Epishina, M.A.; Kulikov, A.S.; Ovchinnikov, I.V.; Ananyev, I.V.; Makhova, N.N. Regioselective synthesis of bifuroxanyl systems with the 3-nitrobifuroxanyl core via a one-pot acylation/nitrosation/cyclization cascade. Tetrahedron Lett. 2016, 57, 4268–4272. [Google Scholar] [CrossRef]
- Muravyev, N.V.; Meerov, D.B.; Monogarov, K.A.; Melnikov, I.N.; Kosareva, E.K.; Fershtat, L.L.; Sheremetev, A.B.; Dalinger, I.L.; Fomenkov, I.V.; Pivkina, A.N. Sensitivity of Energetic Materials: Evidence of Thermodynamic Factor on a Large Array of CHNOFCl Compounds. Chem. Eng. J. 2021, 421 Pt 1, 129804. [Google Scholar] [CrossRef]



| Compound | Td 1 [°C] | ρ 2 [g cm−3] | ΩCO 3 [%] | N+O 4 [%] | IS 5 [J] | FS 6 [N] |
|---|---|---|---|---|---|---|
| 3 | 207 | 1.91 | 0 | 62.8 | 6 | 90 |
| 1 | 218 | 2.13 | +21.3 | 65.9 | 2 | 5 |
| 2 | 186 | 2.14 | +12.2 | 65.0 | 3 | 60 |
| RDX | 204 7 | 1.80 | 0 | 81.1 | 8 7 | 140 7 |
| PETN | 181 7 | 1.78 | +15.2 | 78.5 | 3 7 | 70 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhilin, E.S.; Meerov, D.B.; Fershtat, L.L. Potassium (3-Methyl-2-oxido-1,2,5-oxadiazol-4-yl)dinitromethanide. Molbank 2021, 2021, M1301. https://doi.org/10.3390/M1301
Zhilin ES, Meerov DB, Fershtat LL. Potassium (3-Methyl-2-oxido-1,2,5-oxadiazol-4-yl)dinitromethanide. Molbank. 2021; 2021(4):M1301. https://doi.org/10.3390/M1301
Chicago/Turabian StyleZhilin, Egor S., Dmitry B. Meerov, and Leonid L. Fershtat. 2021. "Potassium (3-Methyl-2-oxido-1,2,5-oxadiazol-4-yl)dinitromethanide" Molbank 2021, no. 4: M1301. https://doi.org/10.3390/M1301
APA StyleZhilin, E. S., Meerov, D. B., & Fershtat, L. L. (2021). Potassium (3-Methyl-2-oxido-1,2,5-oxadiazol-4-yl)dinitromethanide. Molbank, 2021(4), M1301. https://doi.org/10.3390/M1301





