3-Isobutyl-5,5,7-tris(3-methylbut-2-en-1-yl)-1-phenyl-1,7-dihydro-4H-indazole-4,6(5H)-dione
Abstract
:1. Introduction
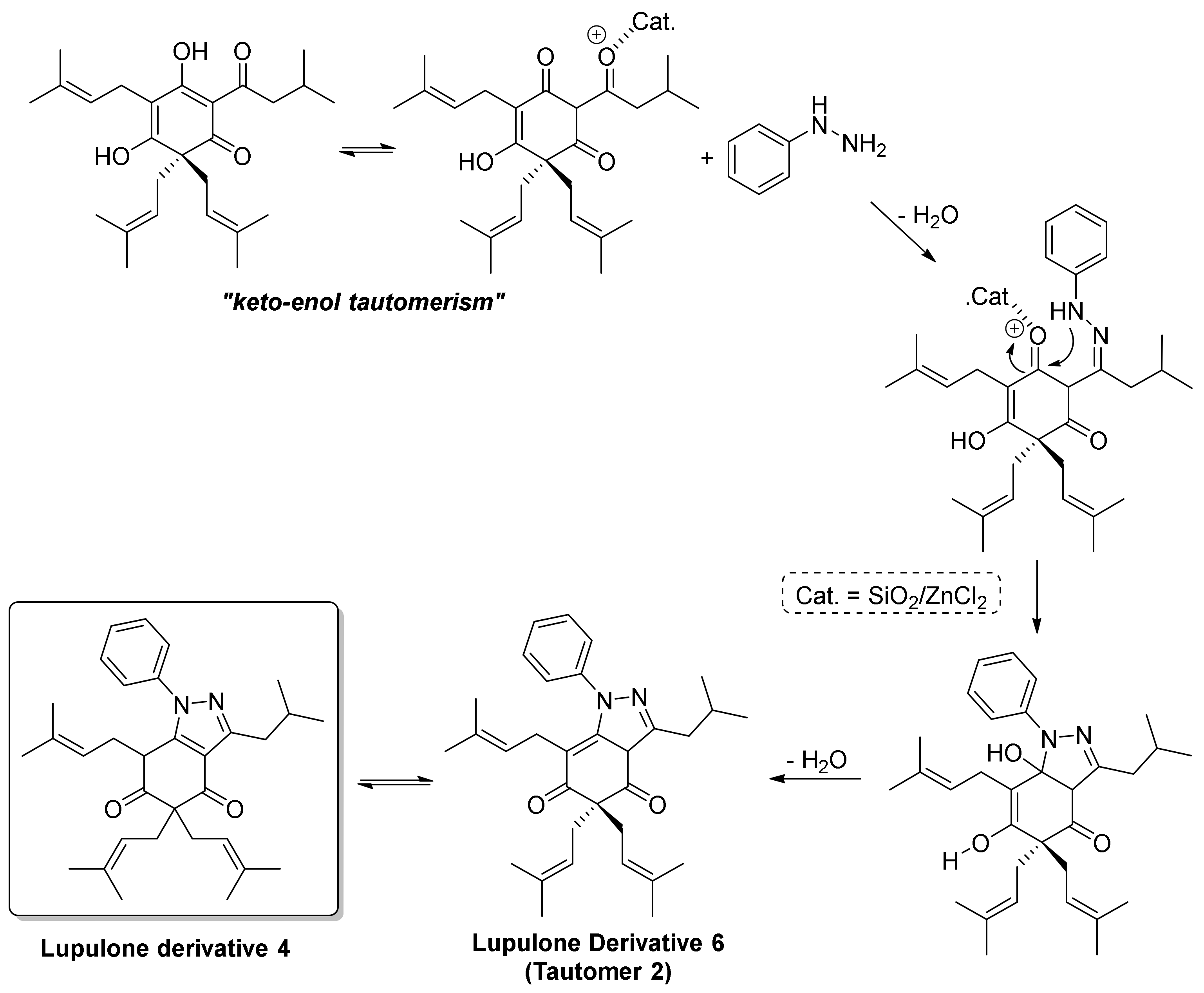
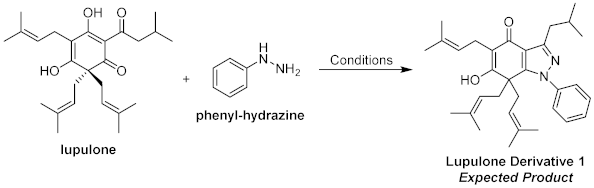
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jacob, R.G.; Oliveira, D.H.; Dias, I.F.C.; Schumacher, R.F.; Savegnago, L. Óleos essenciais como matéria-prima sustentável para o preparo de produtos com maior valor agregado. Rev. Virtual Quim. 2016, 9, 294–316. [Google Scholar] [CrossRef]
- Gallezot, P. Conversion of biomass to selected Chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C.; Chiheb, C.; Pischetsrieder, M. Quantification os co-, n-, and ad-lupulone in hop-based dietary supplements and phytopharmaceuticals and modulation of their contentes by the extraction method. J. Pharm. Biomed. Anal. 2019, 168, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, E.; Archer, R.; Tucknott, M.; Colston, K.; Pirianov, G.; Ramanthan, D.; Dhillon, R.; Sinclair, A.; Skinner, G.A. The synthesis and anticancer effects of a range of natural and unnatural hop β-accids on breast câncer cells. Phytochem. Lett. 2012, 5, 144–149. [Google Scholar] [CrossRef]
- Goese, M.; Kammhuber, K.; Bacher, A.; Zenk, M.H.; Eisenreich, W. Biosynthesis of bitter acids in hops. A 13C-NMR and 2H-NMR study on the building blocks of humulone. Eur. J. Biochem. 1999, 263, 447–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Intelmann, D.; Haseleu, G.; Hofmann, T. LC-MS/MS Quantification of Hop-Derived Bitter Compounds in Beer Using the ECHO Technique. J. Agric. Food Chem. 2009, 57, 1172–1182. [Google Scholar] [CrossRef] [PubMed]
- Tyrrel, E.; Archer, R.; Skinner, G.A.; Singh, K.; Colston, K.; Driver, C. Structure elucidation and an investigation into in vitro effects of hop acids on human cancer cells. Phytochem. Lett. 2010, 3, 17–23. [Google Scholar] [CrossRef]
- Bogdanova, K.; Roderova, M.; Kolar, M.; Langova, K.; Dusek, M.; Jost, P.; Kubelkova, K.; Bostik, P.; Olsovska, J. Antibiofilm activity of bioactive hop compounds humulone, lupulone and xanthohumol toward susceptible and resistant staphylococci. Res. Microbiol. 2018, 169, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.R.; Victoria, F.N.; Oliveira, D.H.; Jacob, R.G.; Savegnago, L.; Alves, D. Essential oil of Psidium cattleianum leaves: Antioxidant and antifungal activity. Pharm. Biol. 2015, 53, 242–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca, A.O.S.; Pereira, D.I.B.; Jacob, R.G.; Maia Filho, F.S.; Oliveira, D.H.; Maroneze, B.P.; Valente, J.S.S.; Osório, L.G.; Botton, S.A.; Meireles, M.C.A. In vitro susceptibility of Brazilian Pythium insidiosum isolates to essential oil of some Lamiaceae Family species. Mycopathologia 2015, 179, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Lenardão, E.J.; Bottesselle, G.V.; Azambuja, F.; Perin, G.; Jacob, R.G. Citronellal as key compounds in organic synthesis. Tetrahedron 2007, 63, 6671–6712. [Google Scholar] [CrossRef]
- Montenegro, L.M.P.; Griep, J.B.; Tavares, F.C.; Oliveira, D.H.; Bianchini, D.; Jacob, R.G. Synthesis and characterization of imine-modified silicas obtained by the reaction of essential oil of Eucalyptus citriodora, 3-aminopropyltriethoxysilane and tetraethylorthosilicate. Vib. Spectrosc. 2013, 68, 272–278. [Google Scholar] [CrossRef]
- Chagas, A.C.; Domingues, L.F.; Fantatto, R.R.; Giglioti, R.; Oliveira, M.C.S.; Oliveira, D.H.; Mano, R.A.; Jacob, R.G. In vitro and in vivo acaricide action of juvenoid analogs produced from the chemical modification of Cymbopogon spp. and Corymbia citriodora essential oil on the cattle tick Rhipicephalus (Boophilus) microplus. Veterin. Parasit. 2014, 205, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.C.; Mano, R.A.; Oliveira, D.H.; Maia, D.S.V.; Silva, W.P.; Savegnago, L.; Lenardão, E.J.; Jacob, R.G. Synthesis, Antimicrobial, and Antioxidant Activities of Chalcogen-Containing Nitrone Derivatives from (R)-Citronellal. Medicines 2017, 4, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taniguchi, Y.; Taniguchi, H.; Yamada, M.; Matsukura, Y.; Koizumi, H.; Furihata, K.; Shindo, K. Analysis of the components of hard resin in hops (Humulus lupulus L.) and structural elucidation of their transformation products formed during the brewing process. J. Agric. Food Chem. 2014, 62, 11602–11612. [Google Scholar] [CrossRef] [PubMed]
- Olsovska, J.; Bostikova, V.; Dusek, M.; Jandovska, V.; Bogdanova, K.; Cermak, P.; Bostik, P.; Mikyska, A.; Kolar, M. Humulus Lupulus L. (Hops)—A valuable source of compounds with bioactive effects for future therapies. Mil. Med. Sci. Lett. 2016, 85, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Radatz, C.S.; Rodrigues, M.B.; Alves, D.; PERIN, G.; Lenardão, E.J.; Savegnago, L.; Jacob, R.G. Synthesis of 1-H-1,5-benzodiazepines derivatives using SiO2/ZnCl2. Heteroat. Chem. 2011, 22, 180–185. [Google Scholar] [CrossRef]
- Pretsch, E.; Bühlman, P.; Badertscher, M. Structure Determination of Organic Compounds, Tables of Spectral Data, 5th ed.; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Briggs, L.H.; Penfold, A.R.; Short, W.F. Leptospermone. Part I. J. Chem. Soc. 1938, 1193–1195. [Google Scholar] [CrossRef]
- Dybowski, M.P.; Typek, R.; Bernacik, K.; Dawidowicz, A.L. Isomerization of bitter acids during the brewing process. Ann. Univesitatis Marie Curie-Sklodowska Lub.-Pol. 2015, 2, 137–144. [Google Scholar] [CrossRef]



| Entry | Catalyst (56 mol %) | Lupulone: Hydrazine Ratio (mmol) | Temp. (°C) | Yield (%) a |
|---|---|---|---|---|
| 1 | -- | (0.5:0.5) | 60 | -- |
| 2 | SiO2/ZnCl2 | (0.5:0.5) | 60 | 51 |
| 3 | SiO2/ZnCl2 | (0.5:0.5) | 25 | 20 |
| 4 | SiO2/ZnCl2 | (0.5:0.5) | 100 | 31 |
| 5 | SiO2/ZnCl2 | (0.5:0.6) | 60 | 75 |
| 6 | SiO2/ZnCl2 | (0.5:0.7) | 60 | 76 |
| Number | 1H (ppm) | 13C (ppm) | 1H-13C HMBC |
|---|---|---|---|
| 1 | --- | --- | --- |
| 2 | --- | --- | --- |
| 3 | --- | 153.6 | --- |
| 4 | --- | 193.1 | --- |
| 5 | --- | 65.7 | --- |
| 6 | --- | 210.0 | --- |
| 7 | 3.89 (dd, J = 5.0 and 6.3 Hz) | 46.8 | 6, 8, 9 and 14 |
| 8 | --- | 147.1 | --- |
| 9 | --- | 118.6 | --- |
| 10 | 2.98 (d, J = 7.2 Hz) | 36.3 | 3 and 9 |
| 11 | 2.25 (n, J = 6.7 Hz) | 28.1 | 3, 10, 12 and 13 |
| 12 | 1.07 (d, J = 6.7 Hz) | 22.4 | 10 and 11 |
| 13 | 1.06 (d, J = 6.7 Hz) | 22.3 | 10 and 11 |
| 14 | 2.10 (ddd, J = 6.3, 6.6 and 14.7 Hz) | 30.1 | 6, 7, 8, 15 and 16 |
| 14’ | 2.55–2.70 (m) | 30.1 | 6, 7, 8, 15 and 16 |
| 15 | 4.60 (t, J = 7.3 Hz) | 118.4 | 14, 17 and 18 |
| 16 | --- | 135.6 | --- |
| 17 | 1.26 (s) | 25.7 | 15 and 16 |
| 18 | 1.53 (s) | 17.4 | 15 and 16 |
| 19 | 2.78 (d, J = 7.4 Hz) | 33.9 | 4, 5, 6, 20 and 21 |
| 20 | 4.93 (t, J = 7.4 Hz) | 118.1 | 19, 22 and 23 |
| 21 | --- | 134.4 | --- |
| 22 | 1.66 (s) | 26.0 | 19, 20 and 21 |
| 23 | 1.72 (s) | 18.0 | 19, 20 and 21 |
| 24 | 2.55–2.70 (m) | 39.5 | 4, 5, 6, 25 and 26 |
| 25 | 4.94 (t, J = 7.3 Hz) | 118.6 | 24, 27 and 28 |
| 26 | --- | 135.1 | --- |
| 27 | 1.53 (s) | 25.9 | 24, 25 and 26 |
| 28 | 1.67 (s) | 17.7 | 24, 25 and 26 |
| 29 | --- | 139.2 | --- |
| 30 | 7.48–7.50 (m) | 124.6 | 29 and 32 |
| 31 | 7.57–7.59 (m) | 129.6 | 29 |
| 32 | 7.53–7.54 (m) | 128.7 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, J.E.R.d.; Hartwig, D.; Jacob, R.G.; Silva, M.S. 3-Isobutyl-5,5,7-tris(3-methylbut-2-en-1-yl)-1-phenyl-1,7-dihydro-4H-indazole-4,6(5H)-dione. Molbank 2022, 2022, M1330. https://doi.org/10.3390/M1330
Nascimento JERd, Hartwig D, Jacob RG, Silva MS. 3-Isobutyl-5,5,7-tris(3-methylbut-2-en-1-yl)-1-phenyl-1,7-dihydro-4H-indazole-4,6(5H)-dione. Molbank. 2022; 2022(1):M1330. https://doi.org/10.3390/M1330
Chicago/Turabian StyleNascimento, José Edmilson Ribeiro do, Daniela Hartwig, Raquel Guimarães Jacob, and Márcio Santos Silva. 2022. "3-Isobutyl-5,5,7-tris(3-methylbut-2-en-1-yl)-1-phenyl-1,7-dihydro-4H-indazole-4,6(5H)-dione" Molbank 2022, no. 1: M1330. https://doi.org/10.3390/M1330
APA StyleNascimento, J. E. R. d., Hartwig, D., Jacob, R. G., & Silva, M. S. (2022). 3-Isobutyl-5,5,7-tris(3-methylbut-2-en-1-yl)-1-phenyl-1,7-dihydro-4H-indazole-4,6(5H)-dione. Molbank, 2022(1), M1330. https://doi.org/10.3390/M1330






