Synthesis, Crystal Structure and Anti-Leukemic Activity of 1,3-Dihydro-1-{1-[4-(4-phenylpyrrolo[1,2-a]quinoxalin-3-yl)benzyl]piperidin-4-yl}-2H-benzimidazol-2-one
Abstract
:1. Introduction
2. Results and Discussion
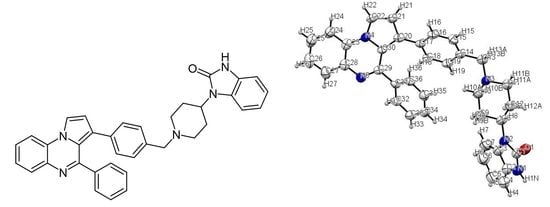
2.1. 1,3-Dihydro-1-{1-[4-(4-phenylpyrrolo[1,2-a]quinoxalin-3-yl)benzyl]piperidin-4-yl}-2H-benzimidazol-2-one
2.2. Cytotoxic Activity
3. Materials and Methods
3.1. 4-(4-Phenylpyrrolo[1,2-a]quinoxalin-3-yl)benzaldehyde (8)
3.2. 1,3-Dihydro-1-{1-[4-(4-phenylpyrrolo[1,2-a]quinoxalin-3-yl)benzyl]piperidin-4-yl}-2H-benzimidazol-2-one (9)
3.3. X-ray Data
3.4. Cytotoxic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalinin, A.A.; Islamova, L.N.; Fazleeva, G.M. New achievements in the synthesis of pyrrolo[1,2-a]quinoxaline. Chem. Heterocycl. Compd. 2019, 55, 584–597. [Google Scholar] [CrossRef]
- Huang, A.; Ma, C. Recent progress in biological activities and synthetic methodologies of pyrroloquinoxalines. Mini-Rev. Med. Chem. 2013, 13, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Campiani, G.; Butini, S.; Fattorusso, C.; Trotta, F.; Franceschina, S.; De Angelis, M.; Nielsen, K.S. Novel Aryl Piperazine Derivatives with Medical Utility. 2006, p. WO2006072608. Available online: https://worldwide.espacenet.com/publicationDetails/originalDocument?CC=WO&NR=2006072608A2&KC=A2&FT=D&ND=3&date=20060713&DB=&locale=fr_EP (accessed on 13 January 2022).
- Campiani, G.; Aiello, F.; Fabbrini, M.; Morelli, E.; Ramunno, A.; Armaroli, S.; Nacci, V.; Garofalo, A.; Greco, G.; Novellino, E.; et al. Quinoxalinylethylpyridylthioureas (QXPTs) as potent non-nucleoside HIV-1 reverse transcriptase (RT) inhibitors. Further SAR studies and identification of a novel orally bioavailable hydrazine-based antiviral agent. J. Med. Chem. 2001, 44, 305–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schann, S.; Mayer, S.; Gardan, S. Pyrrolo[1,2-a]quinoxaline Derivatives as Adenosine A3 Receptor Modulators and Uses Thereof. 2007, p. EP1798233. Available online: https://worldwide.espacenet.com/publicationDetails/biblio?II=0&ND=3&adjacent=true&locale=fr_EP&FT=D&date=20070620&CC=EP&NR=1798233A1&KC=A1 (accessed on 13 January 2022).
- Wang, T.; Tang, Y.; Yang, Y.; An, Q.; Sang, Z.; Yang, T.; Liu, P.; Zhang, T.; Deng, Y.; Luo, Y. Discovery of novel anti-tuberculosis agents with pyrrolo[1,2-a]quinoxaline-base scaffold. Bioorg. Med. Chem. Lett. 2018, 28, 2084–2090. [Google Scholar] [CrossRef] [PubMed]
- Makane, V.B.; Vamshi Krishna, E.; Karale, U.B.; Babar, D.A.; Kalari, S.; Rekha, E.M.; Shukla, M.; Kaul, G.; Sriram, D.; Chopra, S.; et al. Synthesis of novel4,5-dihydropyrrolo[1,2-a]quinoxalines, pyrrolo[1,2-a]quinoxalin-2-ones and their antituberculosis and anticancer activity. Arch. Pharma. 2020, 353, e2000192. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Grellier, P.; Labaied, M.; Sonnet, P.; Léger, J.-M.; Déprez-Poulain, R.; Forfar-Bares, I.; Dallemagne, P.; Lemaître, N.; Péhourcq, F.; et al. Synthesis, antimalarial activity and molecular modeling of new pyrrolo[1,2-a]quinoxalines, bispyrrolo[1,2-a]quinoxalines, bispyrido[3,2-e]pyrrolo[1,2-a]pyrazines and bispyrrolo[1,2-a]thieno[3,2-e]pyrazines. J. Med. Chem. 2004, 47, 1997–2009. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Forfar, I.; Mamani-Matsuda, M.; Desplat, V.; Saliège, M.; Thiolat, D.; Massip, S.; Tabourier, A.; Léger, J.-M.; Dufaure, B.; et al. Synthesis, Analytical Behaviour and Biological Evaluation of New 4-Substituted Pyrrolo[1,2-a]quinoxalines as Antileishmanial Agents. Bioorg. Med. Chem. 2007, 15, 194–210. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Moreau, S.; Ronga, L.; Basmaciyan, L.; Cohen, A.; Rubio, S.; Bentzinger, G.; Azas, N.; Mullié, C.; Sonnet, P. Design, Synthesis and Antimalarial Activity of Some New Aminoalcohol-pyrrolo[1,2-a]quinoxaline Derivatives. Lett. Drug Des. Discov. 2016, 13, 932–942. [Google Scholar] [CrossRef]
- Guillon, J.; Moreau, S.; Mouray, E.; Sinou, V.; Forfar, I.; Belisle-Fabre, S.; Desplat, V.; Millet, P.; Parzy, D.; Jarry, C.; et al. New Ferrocenic Pyrrolo[1,2-a]quinoxaline Derivatives: Synthesis, and In Vitro Antimalarial Activity. Bioorg. Med. Chem. 2008, 16, 9133–9144. [Google Scholar] [CrossRef]
- Guillon, J.; Mouray, E.; Moreau, S.; Mullié, C.; Forfar, I.; Desplat, V.; Belisle-Fabre, S.; Ravanello, F.; Le-Naour, A.; Pinaud, N.; et al. New Ferrocenic Pyrrolo[1,2-a]quinoxaline Derivatives: Synthesis, and in Vitro Antimalarial Activity—Part II. Eur. J. Med. Chem. 2011, 46, 2310–2326. [Google Scholar] [CrossRef] [PubMed]
- Ronga, L.; Del Favero, M.; Cohen, A.; Soum, C.; Le Pape, P.; Savrimoutou, S.; Pinaud, N.; Mullié, C.; Daulouede, S.; Vincendeau, P.; et al. Design, Synthesis and Biological Evaluation of Novel 4-Alkapolyenylpyrrolo[1,2-a]quinoxalines as Antileishmanial Agents—Part III. Eur. J. Med. Chem. 2014, 81, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Cohen, A.; Gueddouda, N.M.; Das, R.N.; Moreau, S.; Ronga, L.; Savrimoutou, S.; Basmaciyan, L.; Monnier, A.; Monget, M.; et al. Design, synthesis and antimalarial activity of novel bis{N-[(pyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}amine derivatives. J. Enzyme Inhib. Med. Chem. 2017, 32, 547–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desplat, V.; Vincenzi, M.; Lucas, R.; Moreau, S.; Savrimoutou, S.; Pinaud, N.; Lesbordes, J.; Peyrilles, E.; Marchivie, M.; Routier, S.; et al. Synthesis and evaluation of the cytotoxic activity of novel ethyl 4-[4-(4-substitutedpiperidin-1-yl)]benzyl-phenylpyrrolo[1,2-a]quinoxaline-carboxylate derivatives in myeloid and lymphoid leukemia cell lines. Eur. J. Med. Chem. 2016, 113, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Desplat, V.; Vincenzi, M.; Lucas, R.; Moreau, S.; Savrimoutou, S.; Rubio, S.; Pinaud, N.; Bigat, D.; Enriquez, E.; Marchivie, M.; et al. Synthesis and Antiproliferative Effect of Ethyl 4-[4-(4-Substituted Piperidin-1-yl)]benzylpyrrolo[1,2-a]quinoxalinecarboxylate Derivatives on Human Leukemia Cells. ChemMedChem 2017, 12, 940–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desplat, V.; Geneste, A.; Begorre, M.-A.; Belisle-Fabre, S.; Brajot, S.; Massip, S.; Thiolat, D.; Mossalayi, D.; Jarry, C.; Guillon, J. Synthesis of New Pyrrolo[1,2-a]quinoxaline Derivatives as Potential Inhibitors of Akt Kinase. J. Enzym. Inhib. Med. Chem. 2008, 23, 648–658. [Google Scholar] [CrossRef] [Green Version]
- Desplat Moreau, S.; Gay, A.; Belisle-Fabre, S.; Thiolat, D.; Massip, S.; Macky, G.; Godde, F.; Mossalayi, D.; Jarry, C.; Guillon, J. Synthesis and evaluation of the antiproliferative ctivity of novel pyrrolo[1,2-a]quinoxaline derivatives, potential inhibitors of Akt Kinase. Part II. J. Enzyme Inhib. Med. Chem. 2010, 25, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Savrimoutou, S.; Rubio, S.; Moreau, S.; Pinaud, N.; Marchivie, M.; Desplat, V. 1-Phenyl-8-[[4-(pyrrolo[1,2-a]quinoxalin-4-yl)phenyl]methyl]-1,3,8-triazaspiro[4.5]decan-4-one: Synthesis, Crystal Structure and Anti-leukemic Activity. Molbank 2020, 2020, M1113. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wei, Y.; Yang, Z.; Li, Y.; Liu, Y.; Liu, P. Highly selective C3-H iodination of pyrrolo[1,2-a]quinoxalines. Org. Biomol. Chem. 2021, 19, 5191–5196. [Google Scholar] [CrossRef] [PubMed]
- Supplementary X-ray Crystallographic Data: Cambridge Crystallographic Data Centre, University Chemical Lab, Lensfield Road, Cambridge, CB2 1EW, UK. Available online: https://www.ccdc.cam.ac.uk/ (accessed on 13 January 2022).
- Sheldrick, G.M. SADABS. University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillon, J.; Savrimoutou, S.; Rubio, S.; Desplat, V. 1-Methyl-3-{4-[(4-(2-oxo-2,3-dihydro-1H-benzimidazol-1-yl)piperidin-1-yl)benzyl]}-2-phenylindole. Molbank 2018, 2018, M1023. [Google Scholar] [CrossRef] [Green Version]



| Compound | IC50 Values (μM) a | ||
|---|---|---|---|
| K562 | HL60 | U937 | |
| 9 | 17.0 ± 0.4 | 31.0 ± 0.5 | 8.0 ± 0.3 |
| JG576 | 19.0 ± 0.5 | 2.0 ± 0.4 | 12.0 ± 0.4 |
| A6730 | 17.0 ± 0.3 | 5.5 ± 0.2 | 8.0 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillon, J.; Savrimoutou, S.; Albenque-Rubio, S.; Pinaud, N.; Moreau, S.; Desplat, V. Synthesis, Crystal Structure and Anti-Leukemic Activity of 1,3-Dihydro-1-{1-[4-(4-phenylpyrrolo[1,2-a]quinoxalin-3-yl)benzyl]piperidin-4-yl}-2H-benzimidazol-2-one. Molbank 2022, 2022, M1333. https://doi.org/10.3390/M1333
Guillon J, Savrimoutou S, Albenque-Rubio S, Pinaud N, Moreau S, Desplat V. Synthesis, Crystal Structure and Anti-Leukemic Activity of 1,3-Dihydro-1-{1-[4-(4-phenylpyrrolo[1,2-a]quinoxalin-3-yl)benzyl]piperidin-4-yl}-2H-benzimidazol-2-one. Molbank. 2022; 2022(1):M1333. https://doi.org/10.3390/M1333
Chicago/Turabian StyleGuillon, Jean, Solène Savrimoutou, Sandra Albenque-Rubio, Noël Pinaud, Stéphane Moreau, and Vanessa Desplat. 2022. "Synthesis, Crystal Structure and Anti-Leukemic Activity of 1,3-Dihydro-1-{1-[4-(4-phenylpyrrolo[1,2-a]quinoxalin-3-yl)benzyl]piperidin-4-yl}-2H-benzimidazol-2-one" Molbank 2022, no. 1: M1333. https://doi.org/10.3390/M1333
APA StyleGuillon, J., Savrimoutou, S., Albenque-Rubio, S., Pinaud, N., Moreau, S., & Desplat, V. (2022). Synthesis, Crystal Structure and Anti-Leukemic Activity of 1,3-Dihydro-1-{1-[4-(4-phenylpyrrolo[1,2-a]quinoxalin-3-yl)benzyl]piperidin-4-yl}-2H-benzimidazol-2-one. Molbank, 2022(1), M1333. https://doi.org/10.3390/M1333