Abstract
The crystal structure of N-(2-benzoyl-4,5-dimethoxyphenethyl)-2-phenylacetamide indicates that the compound crystallizes in the monoclinic C2/c space group with eight molecules in the unit cell. The heteroatoms from the amide group form a chain of intermolecular N-H ··· O hydrogen bonds propagating along the b axis. The carbonyl group from the benzoyl substituent participates in short contacts with two H-atoms from the ethyl or phenyl groups.
1. Introduction
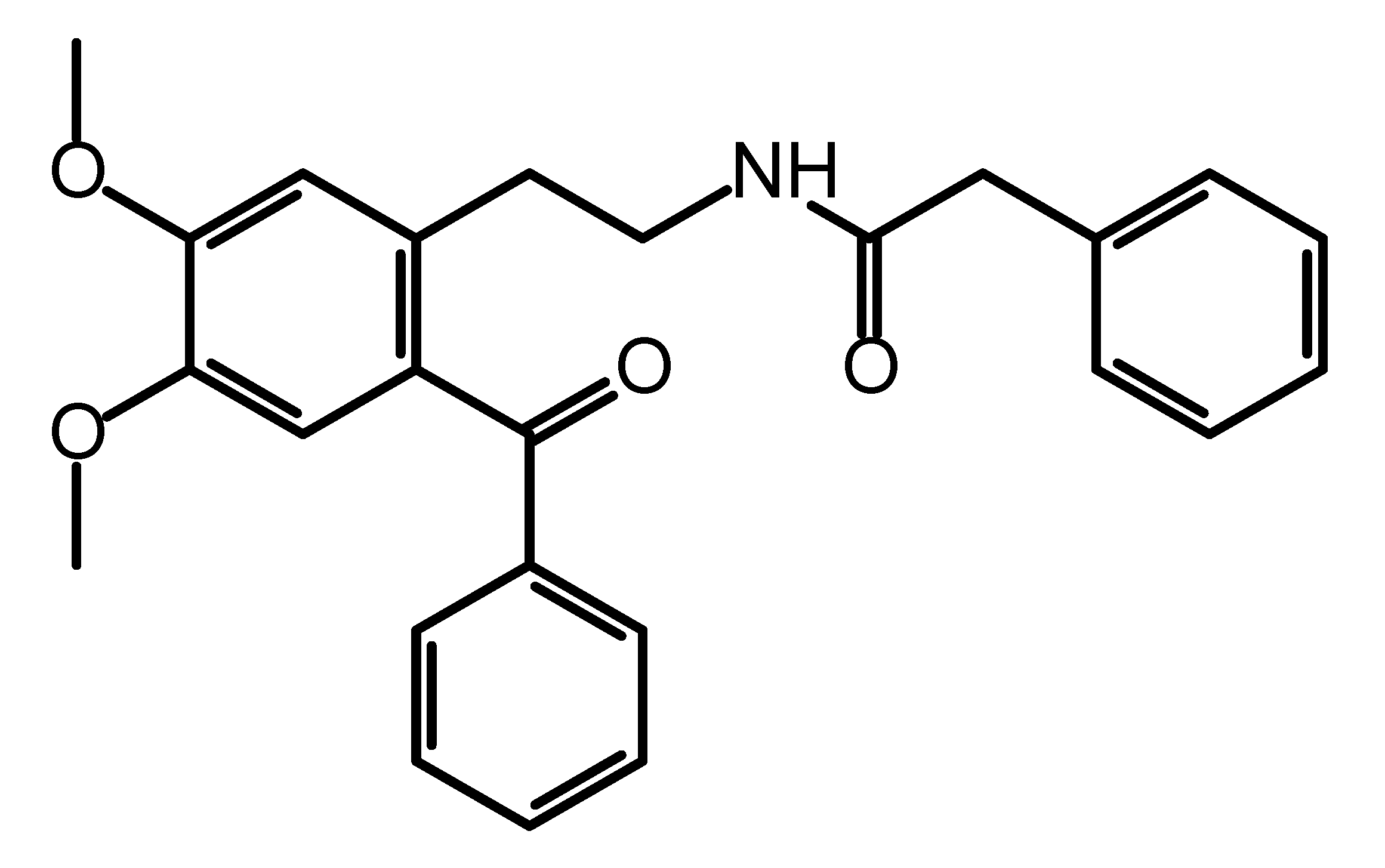
The title compound was synthesized as a precursor for the synthesis of a series of differently substituted 1,2,3,4-tetrahydroisoquinolines and its molecular structure (Figure 1) was described on the basis of spectroscopic data (IR, 1H and 13C NMR) [1]. Besides their use as synthetic scaffolds, 2-phenylacetamides are also known for their application in medicinal chemistry as they possess a variety of biological activities depending on the structural features of the substituents. Their anticonvulsant [2], antidepressant [3] and antiproliferative [4] activities are only a few to mention. Herein, we report the crystal structure of N-(2-benzoyl-4,5-dimethoxyphenethyl)-2-phenylacetamide obtained by single-crystal X-ray diffraction analysis. The presence of suitable coordination groups in the studied ketoamide have provoked systemic study on its coordination properties and some data have recently been published [5]. The data showed that complexation with metal ions is strongly ruled by the coordination ability of the used metal ion. This could be explained with the conformational flexibility of the title compound and its intrinsic ability to form various types of intermolecular hydrogen bonding. In this short note, we provide data on these structural features as observed in the crystal packing.
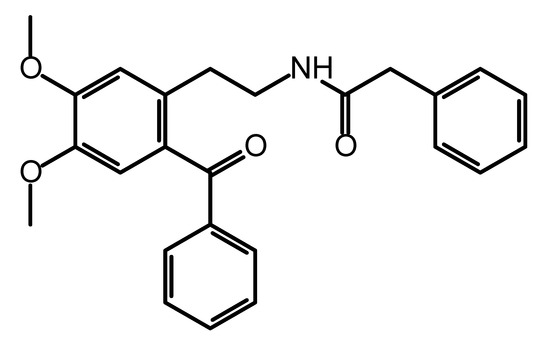
Figure 1.
Molecular structure of N-(2-benzoyl-4,5-dimethoxyphenethyl)-2-phenylacetamide.
2. Results
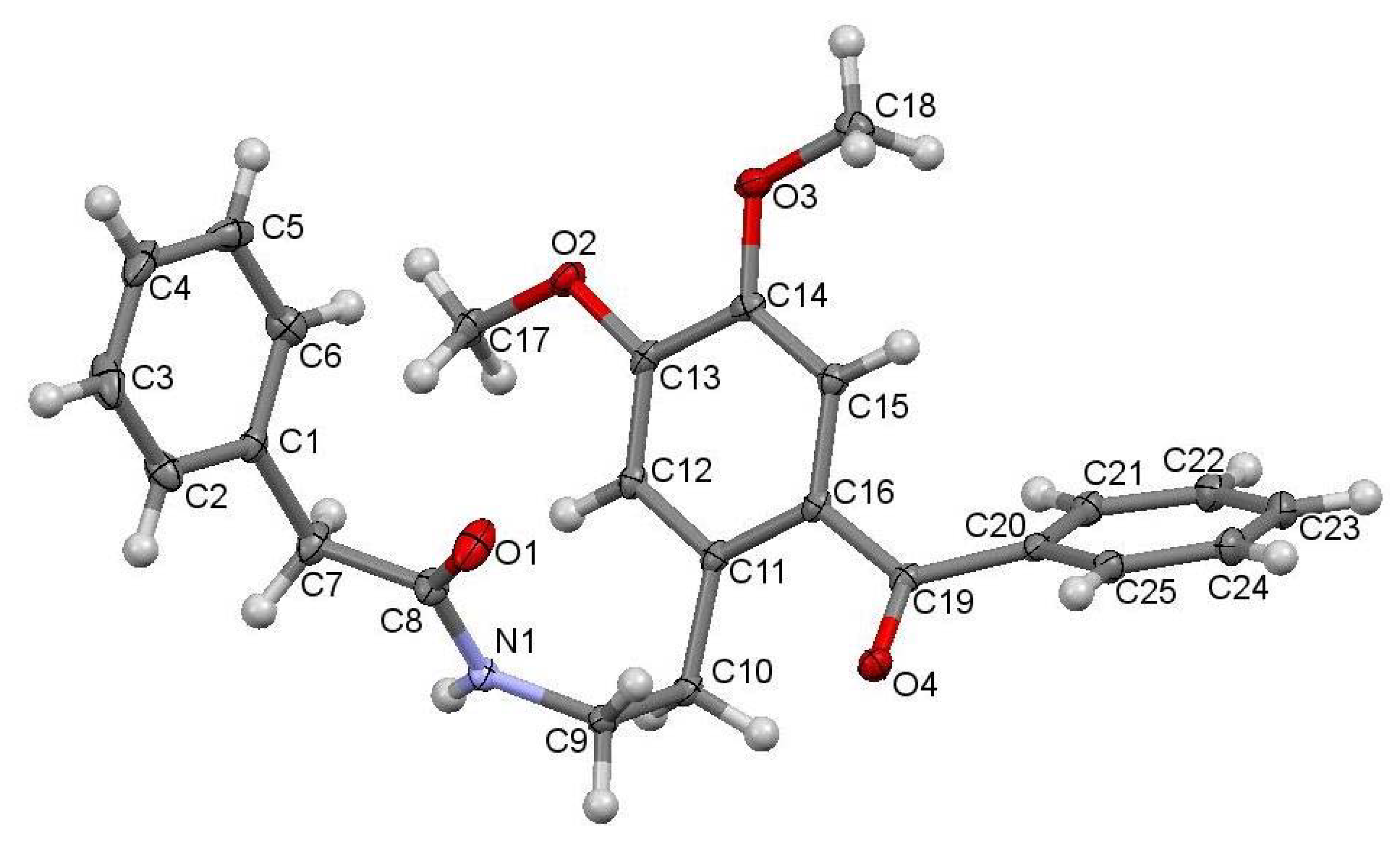
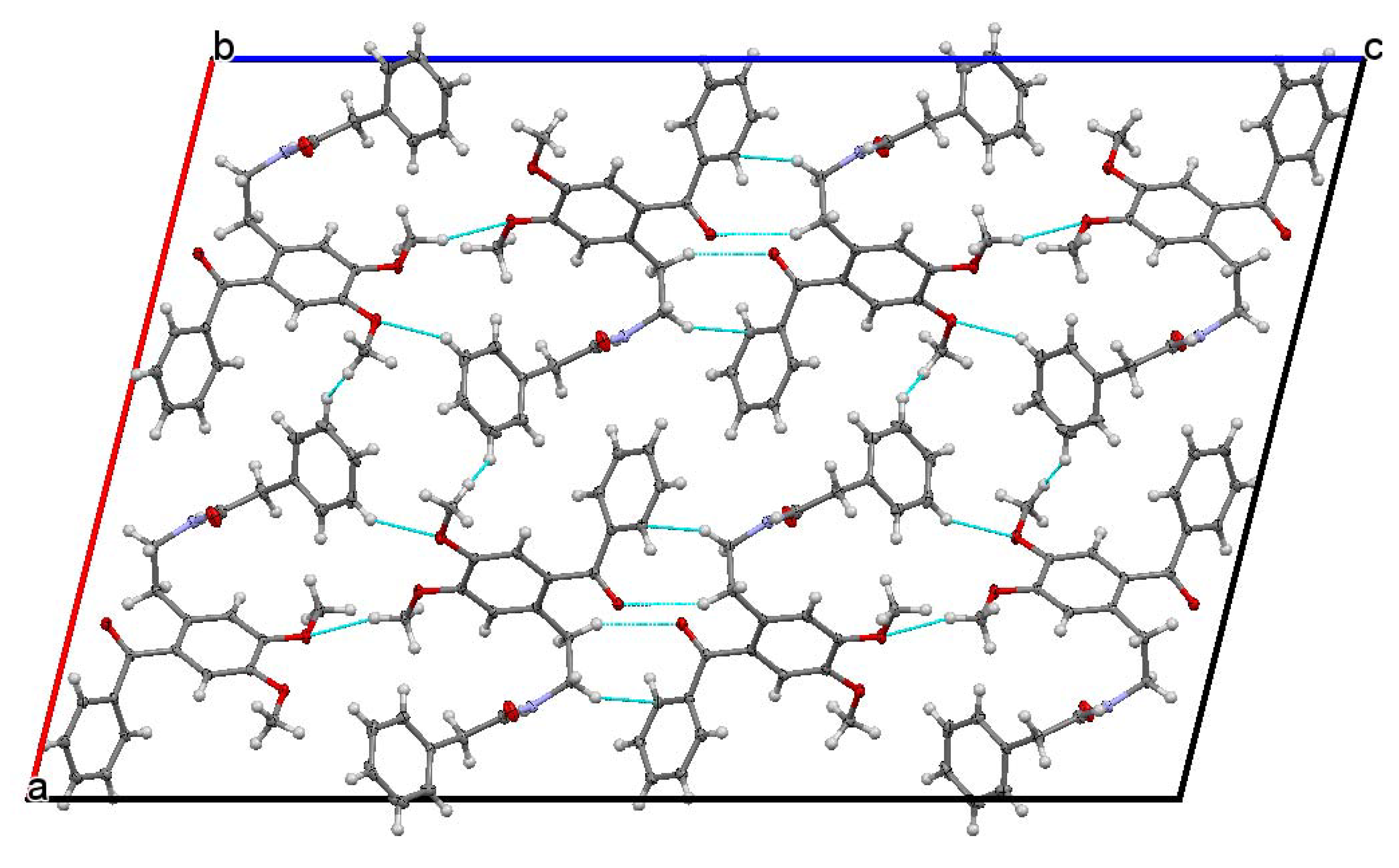
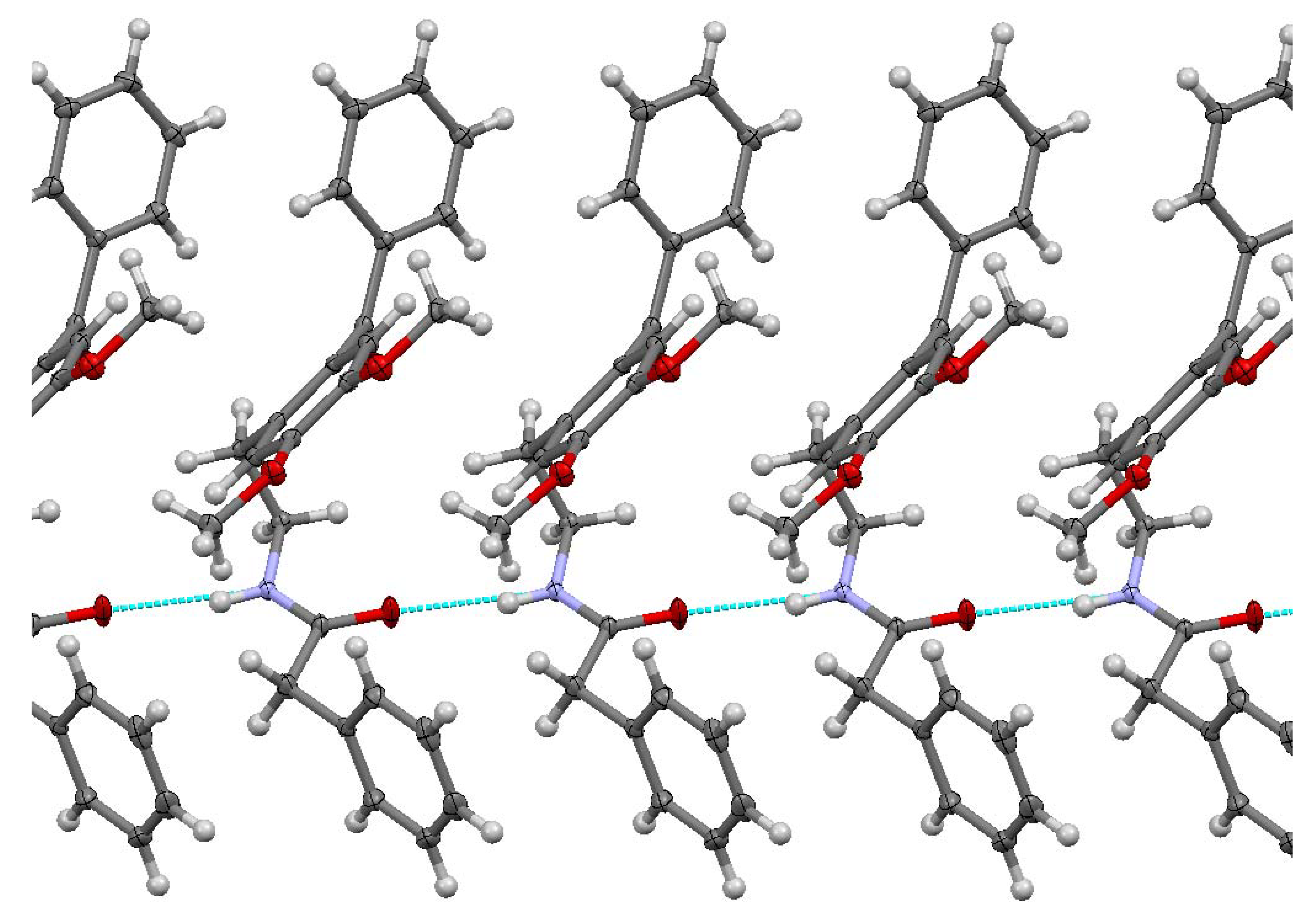
The studied ketoamide compound crystallizes in the monoclinic C2/c space group. The structure of the asymmetric unit is depicted in Figure 2, along with the atom numbering. All three aromatic rings in the molecule are almost orthogonal to each other. The unit cell contains eight molecules and is depicted in Figure 3, where the short contacts between the C-H and C-O groups from adjacent molecules are highlighted. All oxygen atoms participate in the formation of short contacts, whereas the C=O and N-H from the amide group participate in the formation of an intermolecular hydrogen-bonding chain. The propagation of this chain is shown in Figure 4, and the structural features of all hydrogen bonds are summarized in Table 1.
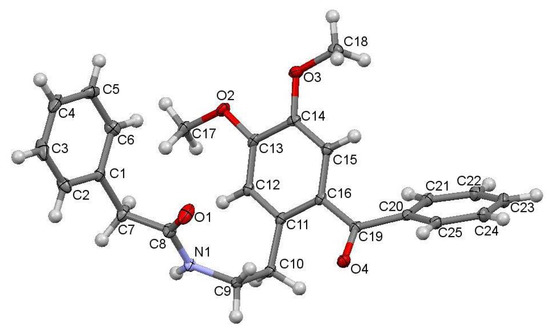
Figure 2.
Crystal structure of N-(2-benzoyl-4,5-dimethoxyphenethyl)-2-phenylacetamide with atoms numbering.
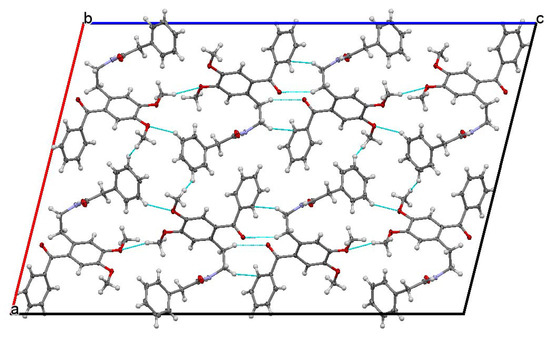
Figure 3.
Unit cell view along the b axis showing the short contacts of C-H…O type.
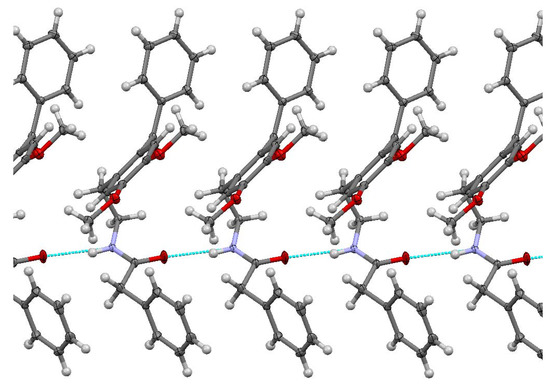
Figure 4.
Chain of C=O…H-N hydrogen bonding for N-(2-benzoyl-4,5-dimethoxyphenethyl)-2-phenylacetamide.

Table 1.
Hydrogen-bond geometry (distances in Å and angles in o) for N-(2-benzoyl-4,5-dimethoxyphenethyl)-2-phenylacetamide.
The title compound has previously been studied for its complexation ability with metal ions (Pd(II) and Zn(II)) and detailed spectroscopic data have been provided [5]. The crystal structure described herein corroborates with the findings from IR, Raman and NMR spectroscopies. The earlier reported IR stretching vibration for the carbonyl groups with frequencies lower than 1700 cm−1 (namely 1662 cm−1 for the C=O from the keto group and 1652 cm−1 for the C=O from the amide group [5]) may suggest that these groups are engaged in moderate intermolecular hydrogen bonding in the solid state, as evidenced by the crystal structure described here.
In summary, the crystal structure of the studied ketoamide reveals the participation of all oxygen atoms in the formation of either intermolecular hydrogen bonding of C=O…H-N type or short contacts with the C-H groups. The latter are CH---π interactions and are quite common in systems featuring aromatic rings. Positively charged hydrogen atoms willingly interact with the electron densities above and below the planes of aromatic rings and are part of Van der Waals interactions.
3. Experimental Section
Good quality single crystals from the title compound were obtained from DMSO-d6 solution in an NMR tube after 1 month of slow evaporation. Yellow prisms with 0.54 × 0.26 × 0.11 mm were analyzed on a 2-circle diffractometer STOE IPDS 2T using GeniX Mo, 0.05 × 0.05 mm2 microfocus as a source for monochromated MoKα radiation (λ = 0.71073 Å) at 120(2) K. Crystal data, data collection and structure refinement details are summarized in Table 2.

Table 2.
Experimental details.
The diffraction data were corrected for absorption effects by the Gaussian integration method implemented in the STOE X-Red32 software. Unit cell parameters were calculated and refined from the full data set. The structures were solved by a full-matrix least squares procedure based on F2 using the SHELX–2014 program package [6], implemented in the Olex [7] and Wingx [8] suites of programs. All non-hydrogen atoms were refined anisotropically, and the positions of the hydrogen atoms were either calculated using a riding model in isotropic approximation or deduced from the electron density map (N-H1). The crystal data have been deposited at the Cambridge Crystallographic Data Centre as CCDC 2157168. (Supplementary Materials). The data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/getstructures. Complete structural parameters for the title compound are listed in Table S1 in the Supplementary Materials along with the 1H and 13C NMR spectra.
Supplementary Materials
The following supporting information can be downloaded online; Table S1: Geometric parameters (bond lengths in Å, and angles in º) for N-(2-benzoyl-4,5-dimethoxyphenethyl)-2-phenylacetamide, along with the cif and check-cif files. 1H and 13C NMR spectra are given in Figures S1 and S2, respectively.
Author Contributions
P.M. and S.N. prepared the compound; A.D. collected the X-ray data and solved the structure; P.M. and S.N. designed the study; A.A. analyzed the data and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The X-ray data are available at CCDC under ref. code CCDC 2157168.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compound are not available.
References
- Ivanov, I.; Nikolova, S.; Aladjov, D.; Stefanova, I.; Zagorchev, P. Synthesis and Contractile Activity of Substituted 1,2,3,4-Tetrahydroisoquinolines. Molecules 2011, 16, 7019–7042. [Google Scholar] [CrossRef] [PubMed]
- Shindikar, A.V.; Viswanathan, C.L. Substituted 2-[2-(pyridin-3-yl) phenyl] acetamides and ureas: Design, synthesis, and anticonvulsant screening in mice. Med. Chem. Res. 2012, 21, 1929–1934. [Google Scholar] [CrossRef]
- Shelke, S.M.; Bhosale, S.H. Synthesis, antidepressant evaluation and QSAR studies of novel 2-(5H-[1, 2, 4] triazino [5, 6-b] indol-3-ylthio)-N-(substituted phenyl) acetamides. Bioorg. Med. Chem. Lett. 2010, 20, 4661–4664. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.L.; Chen, J.J.; Lin, Y.C.; Peng, C.T.; Juang, S.H.; Wang, T.C. Synthesis and antiproliferative activities of N-(naphthalen-2-yl)acetamide and N-(substituted phenyl)acetamide bearing quinolin-2(1H)- one and 3,4-dihydroquinolin-2(1H)-one derivatives. Eur. J. Med. Chem. 2013, 59, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Marinova, P.E.; Tsoneva, S.H.; Nikolova, S.A.; Ivanov, I.I. Novel complexes of N-substituted-4,5-dimethoxy-phenylethyl-2-arylketoamides with metal ions. Bulg. Chem. Commun. 2019, 51, 8–11. [Google Scholar]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).