Synthesis and Ring-Chain Tautomerism of 1-(4-Ethoxyphenyl)-5-formyl-1H-1,2,3-triazole-4-carboxylic Acid: The First Representative of a 5-Formyl-1H-1,2,3-triazole-4-carboxylic Acids Series
Abstract
:1. Introduction
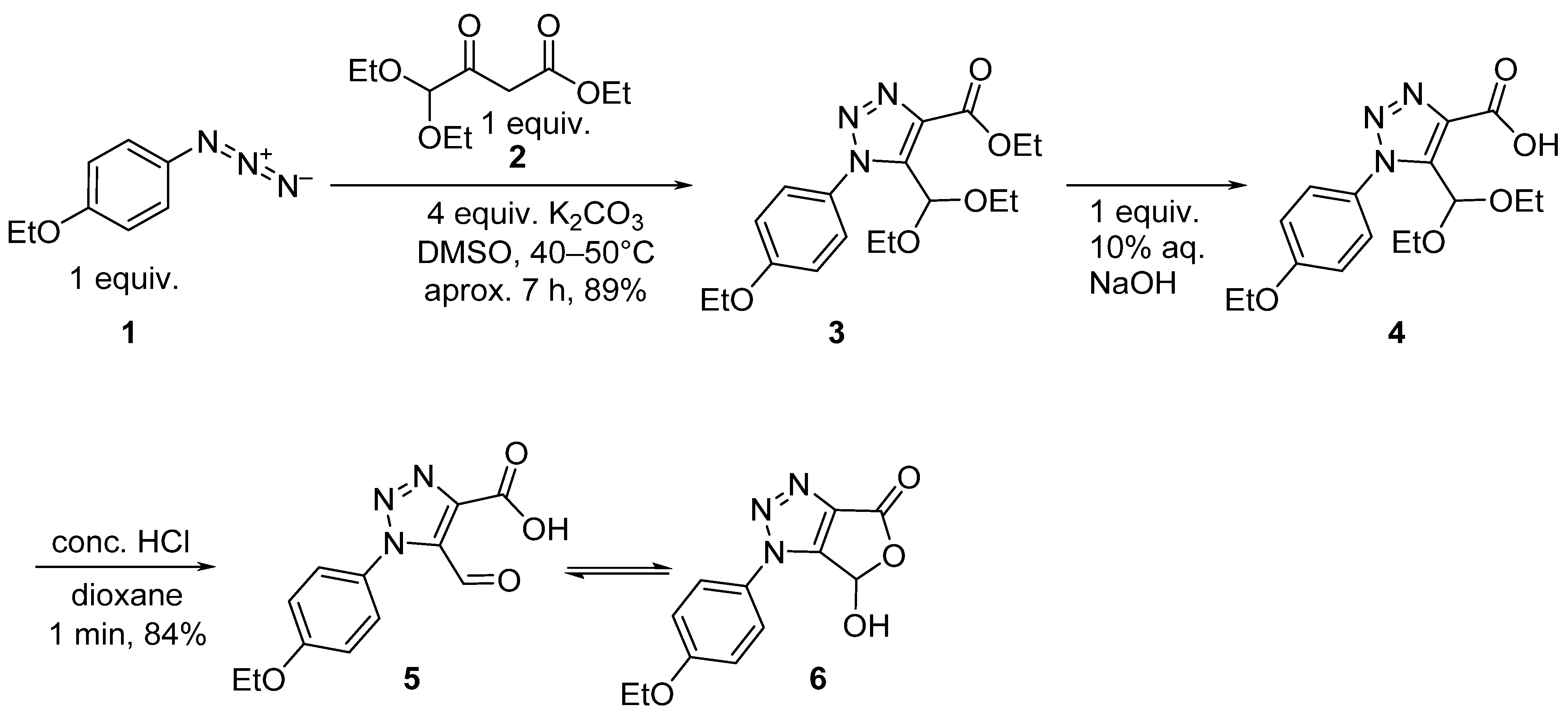
2. Results and Discussion
3. Experimental Section
3.1. Synthesis of 5-(Diethoxymethyl)-1-(4-Ethoxyphenyl)-1H-1,2,3-Triazole-4-Carboxylic Acid 4
3.2. Synthesis of 1-(4-Ethoxyphenyl)-5-Formyl-1H-1,2,3-Triazole-4-Carboxylic Acid 5
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Pokhodylo, N.T.; Shyyka, O.; Matiychuk, V.S.; Obushak, M.D.; Pavlyuk, V.V. A novel base-solvent controlled chemoselective azide attack on an ester group versus keto in alkyl 3-substituted 3-oxopropanoates: Mechanistic insights. Chem. Sel. 2017, 2, 5871–5876. [Google Scholar] [CrossRef]
- Pokhodylo, N.T.; Shyyka, O.Y.; Obushak, M.D. Convenient synthetic path to ethyl 1-aryl-5-formyl-1H-1,2,3-triazole-4-carboxylates and 1-aryl-1,5-dihydro-4H-[1,2,3]triazolo[4,5-d]pyridazin-4-ones. Chem. Heterocycl. Compd. 2018, 54, 773–779. [Google Scholar] [CrossRef]
- Pokhodylo, N.T.; Slyvka, Y.I.; Goreshnik, E.A.; Obushak, M.D. Ethyl 5-formyl-1-(pyridin-3-yl)-1H-1,2,3-triazole-4-carboxylate: Synthesis, Crystal Structure, Hirshfeld Surface Analysis, and DFT Calculation. Molbank 2022, 2022, M1340. [Google Scholar] [CrossRef]
- Konwar, M.; Saikia, M.; Hazarika, R.; Sarma, D. Nickel Chloride Catalyzed Synthesis of Pyrazoles and Phthalazin-1(2H)-ones from Hydrazines at Room Temperature. Tetrahedron Lett. 2022, 98, 153842. [Google Scholar] [CrossRef]
- Kuchlyan, J.; Basak, S.; Dutta, D.; Das, A.K.; Mal, D.; Sarkar, N. A new rhodamine derived fluorescent sensor: Detection of Hg2+ at cellular level. Chem. Phys. Lett. 2017, 673, 84–88. [Google Scholar] [CrossRef]
- Jones, P.R. Ring-Chain Tautomerism. Chem. Rev. 1963, 63, 461–487. [Google Scholar] [CrossRef]
- Freskos, J.N.; Morrow, G.W.; Swenton, J.S. Synthesis of functionalized hydroxyphthalides and their conversion to 3-cyano-1(3H)-isobenzofuranones. The Diels-Alder reaction of methyl 4,4-diethoxybutynoate and cyclohexadienes. J. Org. Chem. 1985, 50, 805–810. [Google Scholar] [CrossRef]
- Behanna, H.A.; Stupp, S.I. Synthesis of stilbene carboxylic acids as scaffolds for calcium sensors. Chem. Commun. 2005, 38, 4845–4847. [Google Scholar] [CrossRef] [PubMed]
- Mal, D.; Pahari, P.; De, S.R. Regiospecific synthesis of 3-(2,6-dihydroxyphenyl)phthalides: Application to the synthesis of isopestacin and cryphonectric acid. Tetrahedron 2007, 63, 11781–11792. [Google Scholar] [CrossRef]
- Pokhodylo, N.T.; Matiychuk, V.S.; Obushak, M.D. Synthesis of isothiocoumarin derivatives. Chem. Heterocycl. Compd. 2010, 46, 140–145. [Google Scholar] [CrossRef]
- Basak, S.; Mandal, S.; Mal, D. First synthetic approach towards K-259-2, a unique calmodulin antagonist. Tetrahedron 2018, 74, 96–103. [Google Scholar] [CrossRef]
- Fedoseev, S.V.; Ershov, O.V.; Lipin, K.V.; Belikov, M.Y. The rare transformation of 2,7-diazaspiro[4.4]nonanes in furo[3,4-c]pyridines. RSC Adv. 2016, 6, 10597–10600. [Google Scholar] [CrossRef]
- Fedoseev, S.V.; Belikov, M.Y.; Ershov, O.V. Synthesis of 3-(Dialkylamino)-4-halofuro[3,4-c]pyridin-1(3H)-ones. Russ. J. Org. Chem. 2020, 56, 49–52. [Google Scholar] [CrossRef]
- Angehrn, P.; Goetschi, E.; Gmuender, H.; Hebeisen, P.; Hennig, M.; Kuhn, B.; Luebbers, T.; Reindl, P.; Ricklin, F.; Schmitt-Hoffmann, A. A New DNA Gyrase Inhibitor Subclass of the Cyclothialidine Family Based on a Bicyclic Dilactam-Lactone Scaffold. Synthesis and Antibacterial Properties. J. Med. Chem. 2011, 54, 2207–2224. [Google Scholar] [CrossRef] [PubMed]
- Pokhodylo, N.T.; Tupychak, M.A.; Obushak, M.D. Metal-Free Synthesis of 1,5-Disubstituted 1,2,3-Triazoles. Russ. J. Org. Chem. 2022, 58, 209–218. [Google Scholar] [CrossRef]
- Priebbenow, D.L.; Zou, L.H.; Becker, P.; Bolm, C. The Disubstitution of Acetals to Prepare δ,δ-Bis (aryl) β-Keto Esters. Eur. J. Org. Chem. 2013, 2013, 3965–3969. [Google Scholar] [CrossRef]
- Hu, M.; Li, J.Q.; Yao, S. In situ “click” assembly of small molecule matrix metalloprotease inhibitors containing zinc-chelating groups. Org. Lett. 2008, 10, 5529–5531. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokhodylo, N.T.; Obushak, M.D. Synthesis and Ring-Chain Tautomerism of 1-(4-Ethoxyphenyl)-5-formyl-1H-1,2,3-triazole-4-carboxylic Acid: The First Representative of a 5-Formyl-1H-1,2,3-triazole-4-carboxylic Acids Series. Molbank 2022, 2022, M1397. https://doi.org/10.3390/M1397
Pokhodylo NT, Obushak MD. Synthesis and Ring-Chain Tautomerism of 1-(4-Ethoxyphenyl)-5-formyl-1H-1,2,3-triazole-4-carboxylic Acid: The First Representative of a 5-Formyl-1H-1,2,3-triazole-4-carboxylic Acids Series. Molbank. 2022; 2022(3):M1397. https://doi.org/10.3390/M1397
Chicago/Turabian StylePokhodylo, Nazariy T., and Mykola D. Obushak. 2022. "Synthesis and Ring-Chain Tautomerism of 1-(4-Ethoxyphenyl)-5-formyl-1H-1,2,3-triazole-4-carboxylic Acid: The First Representative of a 5-Formyl-1H-1,2,3-triazole-4-carboxylic Acids Series" Molbank 2022, no. 3: M1397. https://doi.org/10.3390/M1397
APA StylePokhodylo, N. T., & Obushak, M. D. (2022). Synthesis and Ring-Chain Tautomerism of 1-(4-Ethoxyphenyl)-5-formyl-1H-1,2,3-triazole-4-carboxylic Acid: The First Representative of a 5-Formyl-1H-1,2,3-triazole-4-carboxylic Acids Series. Molbank, 2022(3), M1397. https://doi.org/10.3390/M1397






