Abstract
Bisphosphonates are widely used in medicine and related areas, mainly for the treatment of bone diseases, such as osteoporosis. However, their synthesis is usually performed under harsh reaction conditions. In order to overcome this limitation, the present work illustrates a new synthetic route to access the title α-aminobisphosphonate in milder reaction conditions using α-phosphorylated imines as key intermediates.
1. Introduction
Phosphonate-containing organic molecules such as aminophosphonate and bisphosphonate derivatives are well-known for their biological activities [1,2,3,4,5]. Consequently, many routes have been reported for their synthesis [6,7,8,9,10]. In particular, bisphosphonates have shown high potential as enzyme inhibitors [11,12,13], anti-inflammatory agents [14] or cancer treatments [15,16,17]. In particular, zoledronic acid, considered an essential medicine by the World Health Organization [18,19], and other bisphosphonate analogues, are broadly used for the treatment of osteoporosis and other bone diseases (Figure 1).
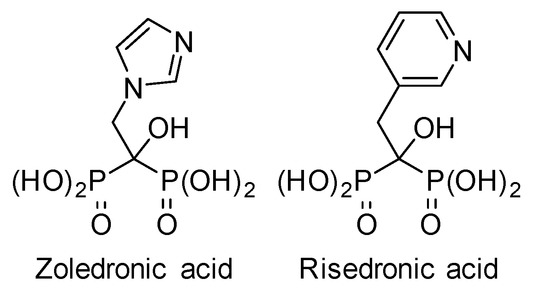
Figure 1.
Bisphosphonate drugs.
Concerning the synthetic routes to α-hydroxy and α-aminobisphosphonates, the most widely used methods make use of carboxylic acids, amides or amines as starting materials (Scheme 1) [6,8]. However, these methodologies often require harsh reaction conditions or high temperatures.
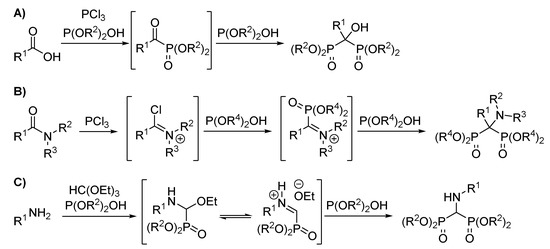
Scheme 1.
Main synthetic routes to bisphosphonates. (A) Synthesi of α hydroxy bisphosphonates from car-boxylic acids; (B) Synthesis of α aminobisphosphonates from amides; (C) Synthesis of α amino bisphosphonates from amines.
In this context, α-iminophosphonates have emerged as promising substrates to access α-aminophosphonate derivatives [20,21] that allow phosphorylated imine intermediates and make them suitable for subsequent transformations [22,23,24]. It should be noted that all those methodologies require milder reaction conditions when compared with the previously known strategies (Scheme 1B,C). Over the last decade, our group has been working on the synthesis of organophosphorus compounds [22,25,26,27,28,29,30]. Based on Steglich’s [31] and Kobayashi’s reports [32,33,34], as well as our previous experience with nucleophilic additions to imines [22,26,28,35,36], in this case, we propose the synthesis of chiral diethyl (benzami-do (diisopropoxyphosphoryl) methyl) phosphonate through a Pudovik reaction using α-bromo aminophosphonates as the starting material of the corresponding imine (Scheme 2).
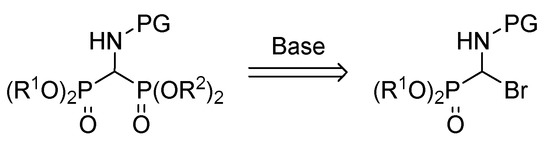
Scheme 2.
Our proposal to access amino bisphosphonates.
2. Results
The proposed synthesis requires two base-catalyzed steps. For this reason, we initially tested the direct addition of diethyl phosphite 2 to α-bromo aminophosphonate 1 in presence of an excess of triethylamine, which is known to promote the elimination of hydrobromic acid as well as to act as a catalyst in the Pudovik reaction. In order to demonstrate the formation of the α-iminophosphonate intermediate 4, the reaction was monitored by 31P NMR (31P NMR of α-iminophosphonate 4 (δ = 1.7 ppm)). The addition of the phosphite 2 with 0.1 equivalents of trimethylamine afforded the bisphosphonate product 3. However, the low stability of α-iminophosphonates makes this two-step procedure less efficient. Therefore, the synthesis of 3 was performed using a single-step procedure, affording the bisphosphonate 3 in 76% yield after purification (Scheme 3).
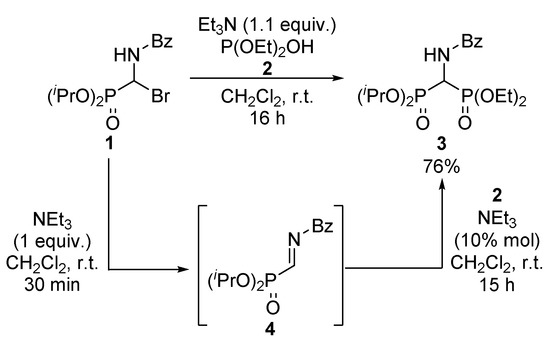
Scheme 3.
Synthesis of diethyl (benzamido (diisopropoxyphosphoryl) methyl) phosphonate 3.
Aminobisphosphonate 3 was extensively characterized by 1H, 13C{1H] NMR, DEPT, 31P NMR, 2D-COSY NMR {1H-1H}, 2D-HSQC NMR {1H-13C}, 2D-HMBC NMR {1H-13C}, FTIR spectra and HRMS experiments (Supplementary Materials).
The most relevant signal of compound 3 in the 1H NMR spectrum (CDCl3) is the proton corresponding to the P-CH-P moiety, which is seen as a representative triplet doublet at δH = 5.19 ppm (2JPH = 21.5 Hz and 3JHH = 10.2 Hz), the result of the coupling between CH and the NH of the amide moiety and the coupling of CH with each of the contiguous phosphonates. In addition, due to the low interchange ratio of the NH belonging to the amide group, a doublet is observed at 6.54 ppm, showing coupling only with the neighboring CH moiety (3JHH = 10.2 Hz). Likewise, in the 13C NMR spectrum of phosphorylated derivative 3, the two doublets corresponding to the two diastereotopic CH carbons of the iso-propyl moiety appear at δC = 72.8 ppm (2JCP = 17.4 Hz) and δC = 72.7 ppm (2JCP = 17.7 Hz). The methylene carbons corresponding to the two ethoxy groups can be also detected as two doublets with chemical shifts at δC = 63.7 ppm (d, 2JCP = 29.3 Hz) and δC = 63.6 ppm (d, 2JCP = 29.7 Hz). A very characteristic signal corresponding to the CH carbon appears as a double doublet at δC = 44.9 ppm with strong coupling with the two adjacent phosphorus atoms (1JCP = 148.4 Hz and 1JCP = 146.6 Hz). Finally, due to the presence of a chiral center in the structure, the two carbons corresponding to the four methyl groups at the isopropyl moieties appear as two doublets at 24.7 ppm (3JCP = 3.3 Hz) and 23.9 ppm (3JCP = 5.4 Hz) for isopropyl groups. However, both methyl groups of the ethoxy group appear overlapped as one doublet at 16.5 ppm (3JCP = 6.0 Hz). As expected, the 31P NMR spectrum of substrate 3 shows two doublets at δP = 16.5 and 14.3 ppm (2JPP = 31.3 Hz).
The FTIR spectrum of compound 3 shows a stretching vibration around ν = 3218 cm−1, which is typical for N-H moiety. In addition, several absorptions within the interval ν = 3056–2986 cm−1 correspond to the stretching vibration of aromatic and aliphatic C-H bonds. One of the most relevant absorption signals observed in the IR spectrum corresponds to the stretching vibration of the amide C=O bond at ν = 1657 cm−1. The vibration of the P=O bonds corresponding to the ethyl and isopropyl phosphonates results in moderate absorption bands at ν = 1258 cm−1 and ν = 1163 cm−1. Due to the presence of the phosphorylated groups, the IR spectrum shows two signals at ν = 1144 cm−1 and ν = 1109 cm−1 which correspond to the P-O-C stretching bonds of both phosphonate moieties.
The high-resolution mass spectrometry (HRMS (ESI-TOF) m/z) experiment shows a peak corresponding to the molecular ion with an exact mass of 436.1642 (M + H)+ that fits with the predicted mass ((M + H)+ = 436.1654) of the calculated molecular formula (C18H32NO7P2) far within the standard tolerated deviation.
3. Materials and Methods
3.1. General Experimental Information
Solvents used for extraction and chromatography were technical grade. All the solvents used in reactions were freshly distilled from appropriate drying agents before use. All other reagents were recrystallized or distilled as necessary. All reactions were performed under an atmosphere of dry nitrogen. Analytical TLC was performed with silica gel 60 F254 plates. Visualization was accomplished by UV light. 1H and 13C-NMR spectra were recorded on a Varian Unity Plus (Varian Inc., NMR Systems, Palo Alto, Santa Clara, CA, USA) (at 300 MHz, 75 MHz, 120 MHz and 282 MHz) and on a Bruker Avance 400 (Bruker BioSpin GmbH, Rheinstetten, Germany) (at 400 MHz for 1H and 100 MHz for 13C). Chemical shifts (δ) were reported in ppm relative to residual CHCl3 (δ = 7.26 ppm for 1H and δ = 77.16 ppm for 13C NMR). Coupling constants (J) were reported in Hertz. Data for 1H NMR spectra were reported as follows: chemical shift, multiplicity, coupling constant and integration. Multiplicity abbreviations were as follows: s = singlet, d = doublet, t = triplet, q = quartet and m = multiplet. 13C-NMR peak assignments were supported by distortionless enhanced polarization transfer (DEPT). High resolution mass spectra (HRMS) were obtained by positive-ion electrospray ionization (ESI). Data were reported in the form m/z (intensity relative to base = 100). Infrared spectra (IR) were taken in a Nicolet iS10 Thermo Scientific spectrometer (Thermo Scientific Inc., Waltham, MA, USA) as neat solids. Peaks were reported in cm−1.
3.2. Experimental Procedures and Characterization Data for Aminobisphosphonate 3
Synthetic procedure: To a solution of 1 (2 mmol, 756 mg, 1.0 equiv.) in dry CH2Cl2 (3 mL) under N2 atmosphere, triethylamine (2.2 mmol, 307 µL, 1.1 equiv.) and diethyl phosphite 2 (2 mmol, 260 µL, 1 equiv.) were sequentially added. Then, the reaction was stirred at room temperature for 16 h, concentrated under vacuum and purified by column chromatography (hexane/EtOAc) to afford 662 mg (76%) of 3 as a white solid.
1H-NMR (400 MHz, CDCl3) δ 7.78 (d, 3JHH = 7.2 Hz, 2H, 2 × CHAr), 7.54 (t, 3JHH = 7.4 Hz, H, CHAr), 7.46 (t, 3JHH = 7.4 Hz, 2H, 2 × CHAr), 6.54 (d, 3JPH = 10.2 Hz, 1H, NH), 5.19 (td, 2JPH = 21.5 Hz, 3JHH = 10.2 Hz, 1H, CH), 4.87–4.74 (m, 2H, 2 × CH OiPr), 4.35–4.11 (m, 4H, 2 × CH2 OEt), 1.65–0.97 (m, 18H, 2 × CH3 OEt + 4 × CH3 OiPr) ppm.
13C-NMR {1H} (101 MHz, CDCl3) δ 166.5 (t, 3JCP = 4.0 Hz, C = O), 133.7 (Cquat), 132.2 (CHAr), 128.9 (2 × CHAr), 127.2 (2 × CHAr), 72.8 (d, 2JCP = 17.4 Hz, CH OiPr), 72.7 (d, 2JCP = 17.7 Hz, CH OiPr), 63.7 (d, 2JCP = 29.3 Hz, CH2 OEt), 63.6 (d, 2JCP = 29.7 Hz, CH2 OEt), 44.9 (dd, 1JCP = 148.4 Hz, 1JCP = 146.6 Hz, CH), 24.7 (d, 3JCP = 3.3 Hz, 2 × CH3 OiPr), 23.9 (d, 3JCP = 5.4 Hz, 2 × CH3 OiPr), 16.5 (d, 3JCP = 6.0 Hz, 2 × CH3 OEt) ppm.
31P-NMR (162 MHz, CDCl3) δ 16.5 (d, 2JPP = 31.3 Hz), 14.3 (d, 2JPP = 31.3 Hz) ppm.
M.p. (Et2O) = 153–155 °C.
FTIR (neat) νmax: 3218 (NH), 3056 (=CH), 2987 (C-H), 1657 (C=O), 1258 (P=O), 1163 (P=O), 1144 (P-O-C), 1109 (P-O-C) cm−1.
HRMS (ESI-TOF) m/z: [M + H] + calcd for C18H32NO7P2 436.1654, Found 436.1642.
4. Conclusions
The synthesis of bisphosphonate derivative 3 was accomplished by the direct addition of diethyl phosphite 2 to a solution of α-bromo aminophosphonate 1 under the presence of an excess of triethylamine. 1H, 13C, 31P and 2D-NMR, and FTIR and HRMS experiments unequivocally confirm the structure of the obtained compound.
Supplementary Materials
The following are available online, 1H, 13C, 31P and 2D-NMR, and FTIR, HRMS and UV spectra copies of compound 3.
Author Contributions
Conceptualization, A.L.-F., F.P., A.M. and J.V.; methodology, A.L.-F. and A.M.; software, A.L.-F. and A.M.; validation, A.M. and J.V.; formal analysis, A.L.-F. and A.M.; investigation, A.L.-F. and A.M.; resources, F.P. and J.V.; data curation, A.L.-F. and A.M.; writing—original draft preparation, A.L.-F. and A.M.; writing—review and editing, A.L.-F., F.P., A.M. and J.V.; visualization, A.M. and J.V.; supervision, A.M. and J.V.; project administration, A.M. and J.V.; funding acquisition, F.P. and J.V. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support by Ministerio de Economía, Industria y Competividad (RTI2018-101818-B-I00) and Gobierno Vasco (GV, IT 992-16) is gratefully acknowledged. A.L.-F. thanks the Basque Country Government for a predoctoral grant.
Data Availability Statement
The data presented in this study are available in the supplementary materials file or on request from the corresponding author (1H, 13C, 31P, 2D-COSY, 2D-HSQC and 2D-HMBC NMR, FTIR, HRMS and HPLC spectra.
Acknowledgments
The authors give thanks for technical and human support provided by SGIker (UPV/EHU/ERDF, EU).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Krečmerová, M.; Majer, P.; Rais, R.; Slusher, B.S. Phosphonates and Phosphonate Prodrugs in Medicinal Chemistry: Past Successes and Future Prospects. Front. Chem. 2022, 10, 889737. [Google Scholar] [CrossRef]
- Kudzin, Z.H.; Kudzin, M.H.; Drabowicz, J.; Stevens, C.V. Aminophosphonic Acids—Phosphorus Analogues of Natural Amino Acids.Part 1: Syntheses of α-Aminophosphonic Acids. Curr. Org. Chem. 2012, 15, 2015–2071. [Google Scholar] [CrossRef]
- Orsini, F.; Sello, G.; Sisti, M. Aminophosphonic Acids and Derivatives. Synthesis and Biological Applications. Curr. Med. Chem. 2009, 17, 264–289. [Google Scholar] [CrossRef]
- Barbosa, J.S.; Braga, S.S.; Almeida Paz, F.A. Empowering the Medicinal Applications of Bisphosphonates by Unveiling Their Synthesis Details. Molecules 2020, 25, 2821. [Google Scholar] [CrossRef]
- Kaboudin, B.; Daliri, P.; Faghih, S.; Esfandiari, H. Hydroxy- and Amino-Phosphonates and -Bisphosphonates: Synthetic Methods and Their Biological Applications. Front. Chem. 2022, 10, 890696. [Google Scholar] [CrossRef]
- Chmielewska, E.; Kafarski, P. Synthetic Procedures Leading towards Aminobisphosphonates. Molecules 2016, 21, 1474. [Google Scholar] [CrossRef]
- Maestro, A.; del Corte, X.; López-Francés, A.; Martinez De Marigorta, E.; Palacios, F.; Vicario, J. Asymmetric Synthesis of Tetrasubstituted α-Aminophosphonic Acid Derivatives. Molecules 2021, 26, 3202. [Google Scholar] [CrossRef]
- Dussart, J.; Deschamp, J.; Migianu-Griffoni, E.; Lecouvey, M. From Industrial Method to the Use of Silylated P(III) Reagents for the Synthesis of Relevant Phosphonylated Molecules. Org. Process Res. Dev. 2020, 24, 637–651. [Google Scholar] [CrossRef]
- Skarpos, H.; Osipov, S.N.; Vorob’Eva, D.V.; Odinets, I.L.; Lork, E.; Röschenthaler, G.V. Synthesis of Functionalized Bisphosphonates via Click Chemistry. Org. Biomol. Chem. 2007, 5, 2361–2367. [Google Scholar] [CrossRef]
- Bortolamiol, E.; Chiminazzo, A.; Sperni, L.; Borsato, G.; Fabris, F.; Scarso, A. Functional Bisphosphonate Synthesis for the Development of New Anti-Resorption Bone Drug Candidates. New J. Chem. 2019, 43, 12641–12649. [Google Scholar] [CrossRef]
- Leung, C.Y.; Langille, A.M.; Mancuso, J.; Tsantrizos, Y.S. Discovery of Thienopyrimidine-Based Inhibitors of the Human Farnesyl Pyrophosphate Synthase—Parallel Synthesis of Analogs via a Trimethylsilyl Ylidene Intermediate. Bioorg. Med. Chem. 2013, 21, 2229–2240. [Google Scholar] [CrossRef]
- Lacbay, C.M.; Mancuso, J.; Lin, Y.S.; Bennett, N.; Götte, M.; Tsantrizos, Y.S. Modular Assembly of Purine-like Bisphosphonates as Inhibitors of HIV-1 Reverse Transcriptase. J. Med. Chem. 2014, 57, 7435–7449. [Google Scholar] [CrossRef]
- Lee, H.F.; Lacbay, C.M.; Boutin, R.; Matralis, A.N.; Park, J.; Waller, D.D.; Guan, T.L.; Sebag, M.; Tsantrizos, Y.S. Synthesis and Evaluation of Structurally Diverse C-2-Substituted Thienopyrimidine-Based Inhibitors of the Human Geranylgeranyl Pyrophosphate Synthase. J. Med. Chem. 2022, 65, 2471–2496. [Google Scholar] [CrossRef]
- Shaddy, A.A.; Kamel, A.A.; Abdou, W.M. Synthesis, Quantitative Structure-Activity Relationship, and Anti-Inflammatory Profiles of Substituted 5-and 6-N-Heterocycle Bisphosphonate Esters. Synth. Commun. 2013, 43, 236–252. [Google Scholar] [CrossRef]
- Monteil, M.; Migianu-Griffoni, E.; Sainte-Catherine, O.; Di Benedetto, M.; Lecouvey, M. Bisphosphonate Prodrugs: Synthesis and Biological Evaluation in HuH7 Hepatocarcinoma Cells. Eur. J. Med. Chem. 2014, 77, 56–64. [Google Scholar] [CrossRef]
- Stresing, V.; Daubiné, F.; Benzaid, I.; Mönkkönen, H.; Clézardin, P. Bisphosphonates in Cancer Therapy. Cancer Lett. 2007, 257, 16–35. [Google Scholar] [CrossRef]
- Coleman, R. The Use of Bisphosphonates in Cancer Treatment. Ann. N. Y. Acad. Sci. 2011, 1218, 3–14. [Google Scholar] [CrossRef]
- WHO World Health Organization. World Health Organization Model List of Essential Medicines: 21st List (2019). 2019. Available online: https://apps.who.int/iris/handle/10665/325771 (accessed on 19 July 2022).
- WHO World Health Organization. World Health Organization Model List of Essential Medicines: 22nd List (2021). 2021. Available online: http://apps.who.int/iris/handle/10665/345533 (accessed on 19 July 2022).
- Maestro, A.; de Marigorta, E.M.; Palacios, F.; Vicario, J. α-Iminophosphonates: Useful Intermediates for Enantioselective Synthesis of α-Aminophosphonates. Asian J. Org. Chem. 2020, 9, 538–548. [Google Scholar] [CrossRef]
- Turcheniuk, K.V.; Kukhar, V.P.; Röschenthaler, G.V.; Aceña, J.L.; Soloshonok, V.A.; Sorochinsky, A.E. Recent Advances in the Synthesis of Fluorinated Aminophosphonates and Aminophosphonic Acids. RSC Adv. 2013, 3, 6693–6716. [Google Scholar] [CrossRef]
- Vicario, J.; Ezpeleta, M.; Palacios, F. Asymmetric Cyanation of α-Ketiminophosphonates Catalyzed by Cinchona Alkaloids: Enantioselective Synthesis of Tetrasubstituted α-Aminophosphonic Acid Derivatives from Trisubstituted α-Aminophosphonates. Adv. Synth. Catal. 2012, 354, 2641–2647. [Google Scholar] [CrossRef]
- Yan, Z.; Wu, B.; Gao, X.; Chen, M.W.; Zhou, Y.G. Enantioselective Synthesis of α-Amino Phosphonates via Pd-Catalyzed Asymmetric Hydrogenation. Org. Lett. 2016, 18, 692–695. [Google Scholar] [CrossRef]
- Inokuma, T.; Sakakibara, T.; Someno, T.; Masui, K.; Shigenaga, A.; Otaka, A.; Yamada, K.I. Asymmetric Synthesis of α-Amino Phosphonic Acids Using Stable Imino Phosphonate as a Universal Precursor. Chem. Eur. J. 2019, 25, 13829–13832. [Google Scholar] [CrossRef]
- Vicario, J.; Ortiz, P.; Palacios, F. Synthesis of Tetrasubstituted α-Aminophosphonic Acid Derivatives from Trisubstituted α-Aminophosphonates. Eur. J. Org. Chem. 2013, 2013, 7095–7100. [Google Scholar] [CrossRef]
- Vicario, J.; Ortiz, P.; Ezpeleta, J.M.; Palacios, F. Asymmetric Synthesis of Functionalized Tetrasubstituted α-Aminophosphonates through Enantioselective Aza-Henry Reaction of Phosphorylated Ketimines. J. Org. Chem. 2015, 80, 156–164. [Google Scholar] [CrossRef]
- Maestro, A.; Martinez De Marigorta, E.; Palacios, F.; Vicario, J. Enantioselective Aza-Reformatsky Reaction with Ketimines. Org. Lett. 2019, 21, 9473–9477. [Google Scholar] [CrossRef]
- Maestro, A.; De Marigorta, E.M.; Palacios, F.; Vicario, J. Enantioselective α-Aminophosphonate Functionalization of Indole Ring through an Organocatalyzed Friedel-Crafts Reaction. J. Org. Chem. 2019, 84, 1094–1102. [Google Scholar] [CrossRef]
- López-Francés, A.; del Corte, X.; Martinez De Marigorta, E.; Palacios, F.; Vicario, J. Ugi Reaction on α-Phosphorated Ketimines for the Synthesis of Tetrasubstituted α-Aminophosphonates and Their Applications as Antiproliferative Agents. Molecules 2021, 26, 1654. [Google Scholar] [CrossRef]
- Maestro, A.; del Corte, X.; Martinez de Marigorta, E.; Palacios, F.; Vicario, J. Enantioselective Synthesis of Functionalized α-Aminophosphonic Acid Derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 287–291. [Google Scholar] [CrossRef]
- Schrader, T.; Kober, R.; Steglich, W. Synthese von 1-Aminophosphonsäure-Derivaten Über Acyliminophosphonsäure-Ester. Synthesis 1986, 5, 372–375. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kiyohara, H.; Nakamura, Y.; Matsubara, R. Catalytic Asymmetric Synthesis of α-Amino Phosphonates Using Enantioselective Carbon-Carbon Bond-Forming Reactions. J. Am. Chem. Soc. 2004, 126, 6558–6559. [Google Scholar] [CrossRef]
- Kiyohara, H.; Nakamura, Y.; Matsubara, R.; Kobayashi, S. Enantiomerically Enriched Allylglycine Derivatives through the Catalytic Asymmetric Allylation of Iminoesters and Iminophosphonates with Allylsilanes. Angew. Chem. Int. Ed. 2006, 45, 1615–1617. [Google Scholar] [CrossRef]
- Kiyohara, H.; Matsubara, R.; Kobayashi, S. High Turnover Frequency Observed in Catalytic Enantioselective Additions of Enecarbamates and Enamides to Iminophosphonates. Org. Lett. 2006, 8, 5333–5335. [Google Scholar] [CrossRef]
- Del Corte, X.; Maestro, A.; Vicario, J.; Martinez De Marigorta, E.; Palacios, F. Brönsted-Acid-Catalyzed Asymmetric Three-Component Reaction of Amines, Aldehydes, and Pyruvate Derivatives. Enantioselective Synthesis of Highly Functionalized γ-Lactam Derivatives. Org. Lett. 2018, 20, 317–320. [Google Scholar] [CrossRef]
- del Corte, X.; López-Francés, A.; Maestro, A.; Martinez de Marigorta, E.; Palacios, F.; Vicario, J. Brönsted Acid Catalyzed Multicomponent Synthesis of Phosphorus and Fluorine-Derived γ-Lactam Derivatives. J. Org. Chem. 2020, 85, 14369–14383. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).