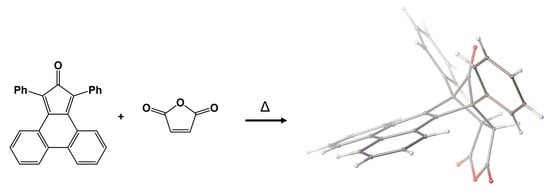
(9R,9aS,12aR,13S)-9,13-Diphenyl-9,9a,12a,13-tetrahydro-9,13-methanotriphenyleno[2,3-c]furan-10,12,14-trione
Abstract
:1. Introduction
2. Results
3. Experimental Section
(9R,9aS,12aR,13S)-9,13-Diphenyl-9,9a,12a,13-tetrahydro-9,13-methanotriphenyleno[2,3-c]furan-10,12,14-trione (3)
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sasaki, T.; Kanematsu, K.; Iizuka, K. Molecular design by cycloaddition reactions XXV. High peri- and regiospecificity of phencyclone. J. Org. Chem. 1976, 41, 1105–1112. [Google Scholar] [CrossRef]
- Carroll, W.R.; Zhao, C.; Smith, M.D.; Pellechia, P.J.; Shimizu, K.D. A molecular balance for measuring aliphatic CH- π interactions. Org. Lett. 2011, 13, 4320–4323. [Google Scholar] [CrossRef]
- Fuchs, B. Photochemical behaviour of bridged compounds. Part II. Photolytic and some ground state reactions of carbonyl-bridged anhydrides and their decarbonylation products. J. Chem. Soc. C 1968, 68–71. [Google Scholar] [CrossRef]
- Zhao, C.; Parrish, R.M.; Smith, M.D.; Pellechia, P.J.; Sherrill, C.D.; Shimizu, K.D. Do deuteriums form stronger CH- π interactions? J. Am. Chem. Soc. 2012, 134, 14306–14309. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Li, P.; Carroll, W.R.; Smith, M.D.; Pellechia, P.J.; Shimizu, K.D. Additivity of Substituent Effects in Aromatic Stacking Interactions. J. Am. Chem. Soc. 2014, 136, 14060–14067. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Li, P.; Smith, M.D.; Pellechia, P.J.; Shimizu, K.D. Experimental study of the cooperativity of CH- π interactions. Org. Lett. 2014, 16, 3520–3523. [Google Scholar] [CrossRef]
- Harrison, E.A. The importance of temperature control in the preparation of phencyclone (1,3-diphenyl-2-H-cyclopenta[I]phenanthrene-2-one). J. Chem. Educ. 1992, 69, 571. [Google Scholar] [CrossRef]
- Smellie, I.A.; Carpenter-Warren, C.L.; Chalmers, B.A.; Cordes, D.B.; De A Gouy, R.P.F.; Keddie, N.S.; Lebl, T.; Patterson, I.L.J.; Slawin, A.M.Z. Simple and inexpensive method for the detection of carbon monoxide released from thermal cheletropic decarbonylation reactions . J. Chem. Educ. 2021, 98, 3608–3613. [Google Scholar] [CrossRef]
- Yit Wooi, G.; White, J.M. Structural Manifestations of the Cheletropic Reaction. Org. Biomol. Chem. 2005, 3, 972–974. [Google Scholar] [CrossRef] [PubMed]
- Hoang, G.T.; Kubo, T.; Young, V.G.; Kautzky, J.A.; Wissinger, J.E. Illustrating the utility of X-ray crystallography for structure elucidation through a tandem aldol Condensation/Diels–Alder reaction sequence. J. Chem. Educ. 2015, 92, 1381–1384. [Google Scholar] [CrossRef]
- Dauben, W.G.; Epstein, W.W. Infrared spectra of some cyclic anhydrides. J. Org. Chem. 1959, 24, 1595–1596. [Google Scholar] [CrossRef]
- Callahan, R.; Ramirez, O.; Rosmarion, K.; Rothchild, R.; Bynum, K.C. Multinuclear NMR Studies and ab initio structure calculations of hindered phencyclone Diels-Alder adducts from symmetrical cyclic dienophiles: Cyclohexene, vinylene carbonate, vinylene trithiocarbonate and two N-aryl maleimides. J. Heterocycl. Chem. 2005, 42, 889–898. [Google Scholar] [CrossRef]
- CrystalClear-SM Expert; v2.1; Commercial software; Rigaku Americas: The Woodlands, TX, USA; Rigaku Corporation: Tokyo, Japan, 2015.
- CrysAlisPro; v1.171.38.46; Commercial software; Rigaku Oxford Diffraction, Rigaku Corporation: Oxford, UK, 2015.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blake, C.; Chalmers, B.A.; Clough, L.A.; Clunie, D.; Cordes, D.B.; Lebl, T.; McDonald, T.R.; Smith, S.R.; Stuart-Morrison, E.; Smellie, I.A. (9R,9aS,12aR,13S)-9,13-Diphenyl-9,9a,12a,13-tetrahydro-9,13-methanotriphenyleno[2,3-c]furan-10,12,14-trione. Molbank 2022, 2022, M1435. https://doi.org/10.3390/M1435
Blake C, Chalmers BA, Clough LA, Clunie D, Cordes DB, Lebl T, McDonald TR, Smith SR, Stuart-Morrison E, Smellie IA. (9R,9aS,12aR,13S)-9,13-Diphenyl-9,9a,12a,13-tetrahydro-9,13-methanotriphenyleno[2,3-c]furan-10,12,14-trione. Molbank. 2022; 2022(3):M1435. https://doi.org/10.3390/M1435
Chicago/Turabian StyleBlake, Corrin, Brian A. Chalmers, Lewis A. Clough, Dylan Clunie, David B. Cordes, Tomas Lebl, Timothy R. McDonald, Siobhan R. Smith, Elliott Stuart-Morrison, and Iain A. Smellie. 2022. "(9R,9aS,12aR,13S)-9,13-Diphenyl-9,9a,12a,13-tetrahydro-9,13-methanotriphenyleno[2,3-c]furan-10,12,14-trione" Molbank 2022, no. 3: M1435. https://doi.org/10.3390/M1435