Benz[a]azulenequinones: Reaction of Benz[a]azulenes with Pyridinium Hydrobromide Perbromide
Abstract
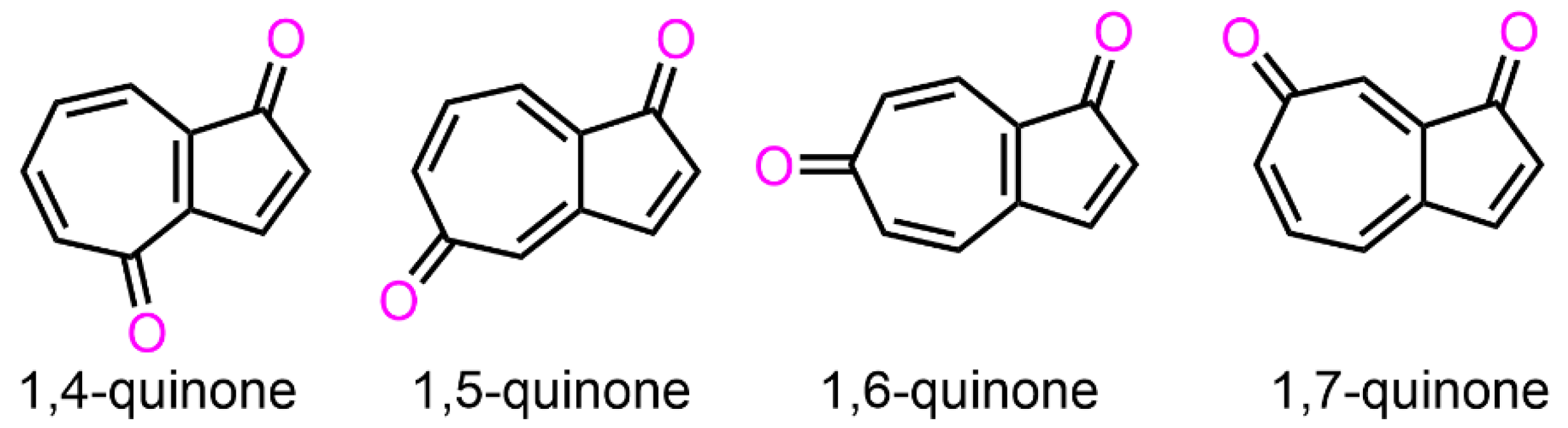
1. Introduction
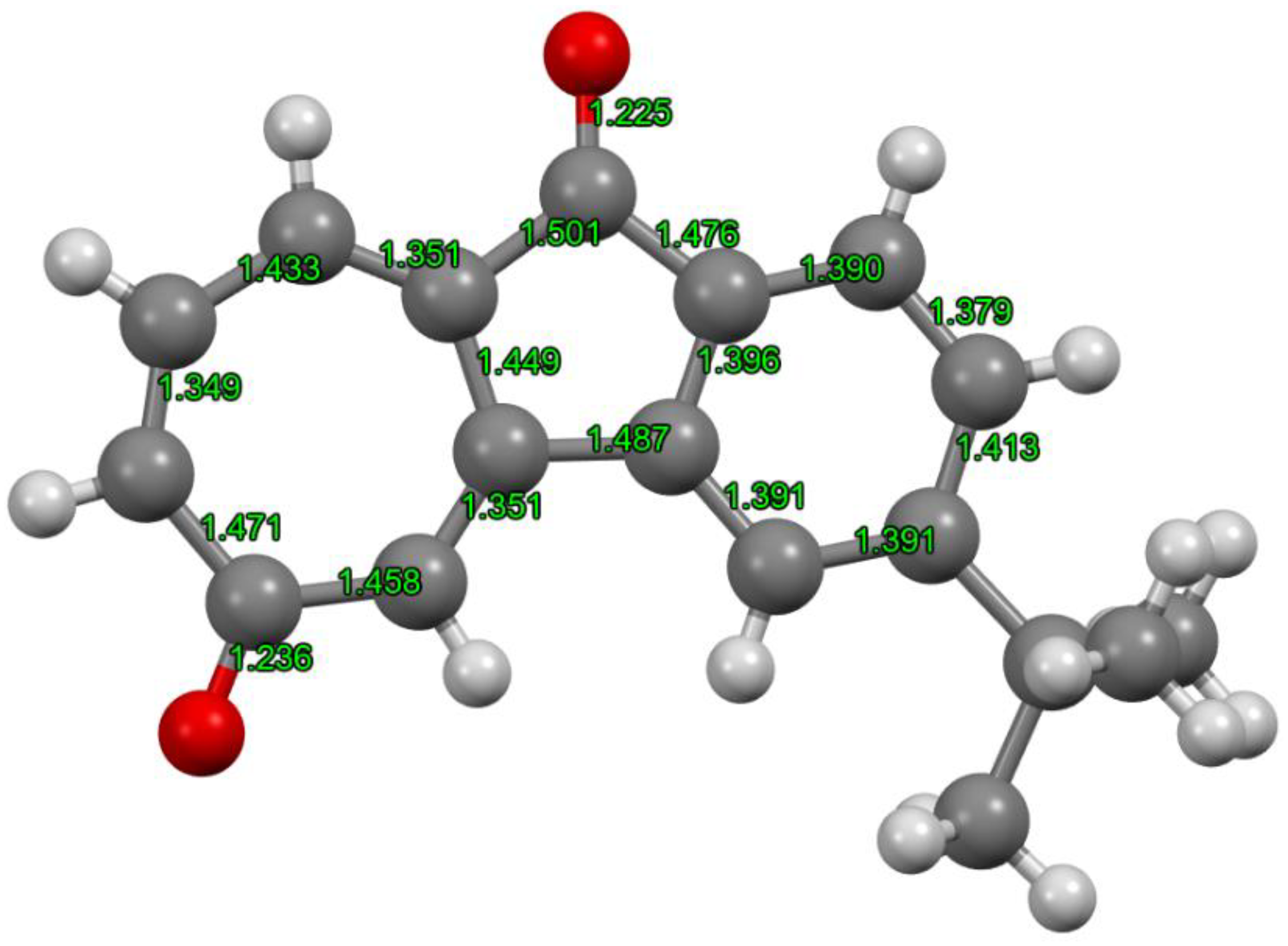
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Scott, L.T.; Rozeboom, M.D.; Houk, K.N.; Fukunaga, T.; Lindner, H.J.; Hafner, K. The quinones of azulene. A theoretical prognosis. J. Am. Chem. Soc. 1980, 102, 5169–5176. [Google Scholar] [CrossRef]
- Morita, T.; Takase, K. The Synthesis of Diethyl 2,6-Dioxoazulene-1,3-Dicarboxylate, A Derivative of 2,6-Azulenedione (2,6-AzulenoQuinone). Chem. Lett. 1977, 6, 513–516. [Google Scholar] [CrossRef]
- Morita, T.; Karasawa, M.; Takase, K. The Synthesis and Some Properties of 1,2-Azulenequinone (1,2-Azulenedione) and its Derivatives. Chem. Lett. 1980, 9, 197–200. [Google Scholar] [CrossRef]
- Scott, L.T.; Griitter, P.; Chamberlain, R.E., III. Quinones of Azulene. 3. Generation and Trapping of the Reactive 1,4- and 1,6-Quinones. J. Am. Chem. Soc. 1984, 106, 4852–4856. [Google Scholar] [CrossRef]
- Scott, L.T.; Adams, C.M. Quinones of Azulene. 4. Synthesis and Characterization of the Parent 1,5- and 1,7-Quinones. J. Am. Chem. Soc. 1984, 106, 4857–4861. [Google Scholar] [CrossRef]
- Nozoe, T.; Wakabayashi, H.; Shindo, K.; Kurihara, T.; Ishikawa, S.; Kageyama, M. A Highly Convenient, One-Pot Synthesis of 3-Bromo-1,5- and -1,7-Azulenequinones by Polybromination of Azulene. Chem. Lett. 1995, 24, 25–26. [Google Scholar] [CrossRef]
- Matsuo, H.; Fujimori, K.; Ohta, A.; Kakehi, A.; Yasunami, M.; Nozoe, T. Synthesis of Azulenequinones Fused with Thiophene and Furan. Heterocycles 2003, 61, 271–279. [Google Scholar] [CrossRef]
- Sigrist, R.; Hansen, H.J. Benzo[a]azulenediones and 10,10′-Bibenzo[a]azulene. Helv. Chim. Acta 2014, 97, 1165–1175. [Google Scholar] [CrossRef]
- Shoji, T.; Yamazaki, A.; Katoh, R.; Shimamura, K.; Sakai, R.; Yasunami, M.; Okujima, T.; Ito, S. Synthesis, Reactivity, and Properties of Benz[a]azulenes via the [8 + 2] Cycloaddition of 2H-Cyclohepta[b]furan-2-ones with an Enamine. J. Org. Chem. 2022, 87, 5827–5845. [Google Scholar] [CrossRef] [PubMed]


| Entry | Substrate | Product | Yield [%] 1 |
|---|---|---|---|
| 1 | 1a | 2a | 24 |
| 2 | 1b | 2b | 42 |
| 3 | 1c | 2c | 43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoji, T.; Yamazaki, A.; Sakata, N.; Sekiguchi, R.; Ito, S.; Yasunami, M. Benz[a]azulenequinones: Reaction of Benz[a]azulenes with Pyridinium Hydrobromide Perbromide. Molbank 2022, 2022, M1467. https://doi.org/10.3390/M1467
Shoji T, Yamazaki A, Sakata N, Sekiguchi R, Ito S, Yasunami M. Benz[a]azulenequinones: Reaction of Benz[a]azulenes with Pyridinium Hydrobromide Perbromide. Molbank. 2022; 2022(4):M1467. https://doi.org/10.3390/M1467
Chicago/Turabian StyleShoji, Taku, Akari Yamazaki, Naoko Sakata, Ryuta Sekiguchi, Shunji Ito, and Masafumi Yasunami. 2022. "Benz[a]azulenequinones: Reaction of Benz[a]azulenes with Pyridinium Hydrobromide Perbromide" Molbank 2022, no. 4: M1467. https://doi.org/10.3390/M1467
APA StyleShoji, T., Yamazaki, A., Sakata, N., Sekiguchi, R., Ito, S., & Yasunami, M. (2022). Benz[a]azulenequinones: Reaction of Benz[a]azulenes with Pyridinium Hydrobromide Perbromide. Molbank, 2022(4), M1467. https://doi.org/10.3390/M1467






