Synthesis of 6-Methyluracilpentylviologen Resorcinarene Cavitand
Abstract
1. Introduction
2. Results
3. Materials and Methods
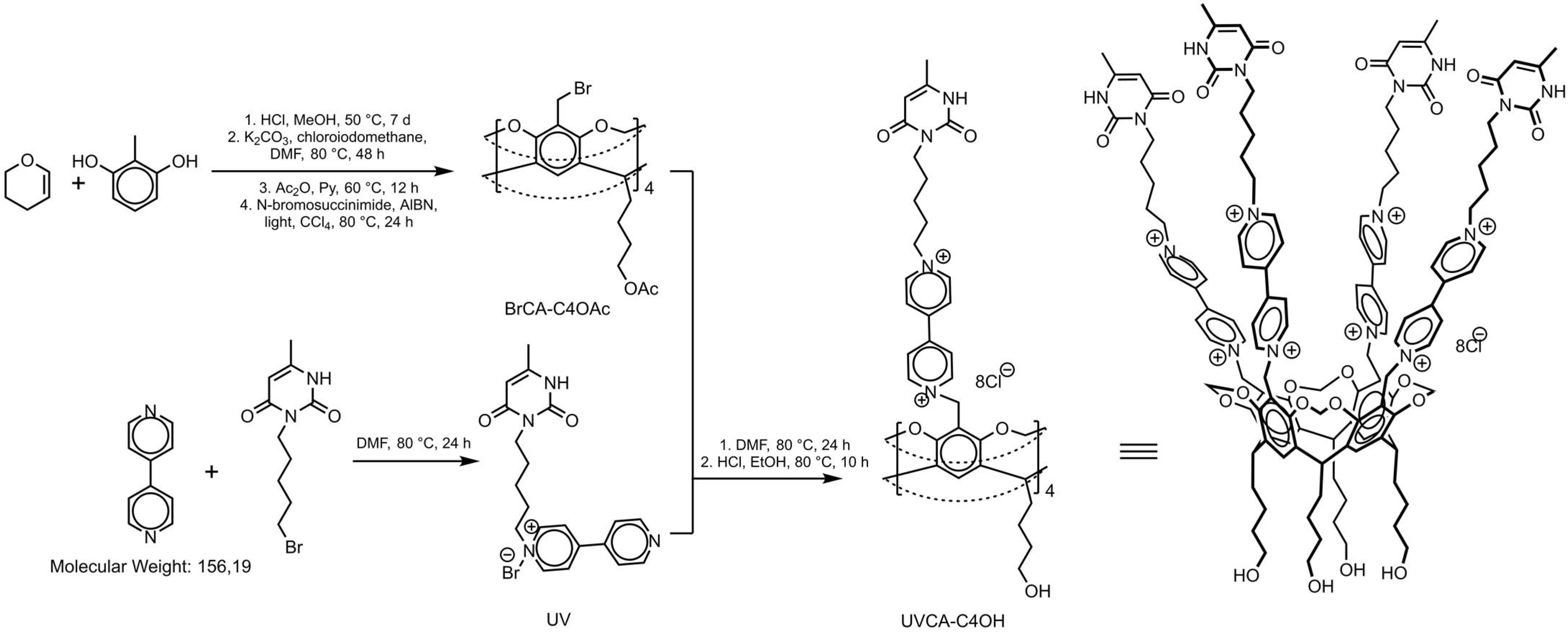
3.1. Synthesis of UV
3.2. Synthesis of UVCA-C4OAc
3.3. Synthesis of UVCA-C4OH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Kenchegowda, M.; Rahamathulla, M.; Hani, U.; Begum, M.Y.; Guruswamy, S.; Osmani, R.; Ali, M.; Gowrav, M.P.; Alshehri, S.; Ghoneim, M.M.; et al. Smart nanocarriers as an emerging platform for cancer therapy: A review. Molecules 2022, 27, 146. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Dai, X.; Wang, X.; Tan, P.; Gu, L.; Luo, Q.; Zheng, X.; Li, Z.; Zhu, H.; Zhang, H. A nanostrategy for efficient imaging-guided antitumor therapy through a stimuli-responsive branched polymeric prodrug. Adv. Sci. 2020, 7, 1903243. [Google Scholar] [CrossRef]
- Perrault, S.D.; Walkey, C.; Jennings, T.; Fischer, H.C.; Chan, W.C. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 2009, 9, 1909–1915. [Google Scholar] [CrossRef]
- Allen, T.M. Ligand-targeted therapeutics in anticancer therapy. Nat. Rev. Cancer 2002, 2, 750–763. [Google Scholar] [CrossRef]
- Bai, H.; Wang, J.; Li, Z.; Tang, G. Macrocyclic compounds for drug and gene delivery in immune-modulating Therapy. Int. J. Mol. Sci. 2019, 20, 2097. [Google Scholar] [CrossRef]
- Zheng, Z.; Geng, W.-C.; Xu, Z.; Guo, D.-S. Macrocyclic amphiphiles for drug delivery. Isr. J. Chem. 2019, 59, 913–927. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Zhao, L.; Zhang, Y.; Chen, L.; Ma, M.; Du, X.; Meng, Z.; Li, C.; Meng, Q. Supramolecular drug delivery system from macrocycle-based self-assembled amphiphiles for effective tumor therapy. ACS Appl. Mater. Interfaces 2021, 13, 53564–53573. [Google Scholar] [CrossRef]
- Yu, J.; Qi, D.; Li, J. Design, synthesis and applications of responsive macrocycles. Commun. Chem. 2020, 3, 189. [Google Scholar] [CrossRef]
- Fan, X.; Guo, X. Development of calixarene-based drug nanocarriers. J. Mol. Liq. 2021, 325, 115246. [Google Scholar] [CrossRef]
- Hoskins, C.; Curtis, A.D.M. Simple calix[n]arenes and calix[4]resorcinarenes as drug solubilizing agents. J. Nanomed. Res. 2015, 2, 65–71. [Google Scholar]
- Shetty, D.; Skorjanc, T.; Olson, M.A.; Trabolsi, A. Self-assembly of stimuli-responsive imine-linked calix[4]arene nanocapsules for targeted camptothecin delivery. Chem. Commun. 2019, 55, 8876–8879. [Google Scholar] [CrossRef] [PubMed]
- Ciepluch, K.; Katir, N.; El Kadib, A.; Felczak, A.; Zawadzka, K.; Weber, M.; Klajnert, B.; Lisowska, K.; Caminade, A.-M.; Bousmina, M.; et al. Biological properties of new viologen-phosphorus dendrimers. Mol. Pharm. 2012, 9, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Bongard, D.; Bohr, W.; Swierczek, M.; Degefa, T.H.; Walder, L.; Brandt, R. Alkylene-bridged viologen dendrimers: Versatile cell delivery tools with biosensing properties. Org. Biomol. Chem. 2014, 12, 9583–9591. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lepadatu, A.-M.; Zhu, Y.; Ciobanu, M.; Wang, Y.; Asaftei, S.C.; Oupický, D. Examination of structure–activity relationship of viologen-based dendrimers as CXCR4 antagonists and gene carriers. Bioconjug. Chem. 2014, 25, 907–917. [Google Scholar] [CrossRef]
- Pałasz, A.; Cież, D. In search of uracil derivatives as bioactive agents. Uracils and fused uracils: Synthesis, biological activity and applications. Eur. J. Med. Chem. 2015, 97, 582–611. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, D.; Vijayakumar, B.G.; Kannan, T. Therapeutic potential of uracil and its derivatives in countering pathogenic and physiological disorders. Eur. J. Med. Chem. 2020, 207, 112801. [Google Scholar] [CrossRef] [PubMed]
- Semenov, V.E.; Zueva, I.V.; Mukhamedyarov, M.A.; Lushchekina, S.V.; Kharlamova, A.D.; Petukhova, E.O.; Mikhailov, A.S.; Podyachev, S.N.; Saifina, L.F.; Petrov, K.A.; et al. 6-Methyluracil derivatives as bifunctional acetylcholinesterase inhibitors for the treatment of Alzheimer’s disease. ChemMedChem 2015, 10, 1863–1874. [Google Scholar] [CrossRef]
- Bossmann, S.; Leaym, X.; Kraft, S. Synthesis of Water-soluble highly charged and methylene-bridged resorcin[4]arenes. Synthesis 2008, 6, 932–942. [Google Scholar] [CrossRef]
- Gibb, B.C.; Chapman, R.G.; Sherman, J.C. Synthesis of hydroxyl-footed cavitands. J. Org. Chem. 1996, 61, 1505–1509. [Google Scholar] [CrossRef]
- Sultanova, E.D.; Mukhitova, R.K.; Ziganshina, A.Y.; Konovalov, A.I.; Krasnova, E.G.; Kharlamov, S.V.; Nasybullina, G.R.; Yanilkin, V.V.; Nizameev, I.R.; Kadirov, M.K.; et al. Thermoresponsive polymer nanoparticles based on viologen cavitands. ChemPlusChem 2015, 80, 217–222. [Google Scholar] [CrossRef]
- Semenov, V.E.; Akamsin, V.D.; Reznik, V.S. Synthesis of acyclic and macrocyclic analogs of di-, tri-, and tetranucleotides. Russ. J. Gen. Chem. 2007, 77, 1430–1440. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziganshina, A.Y.; Mansurova, E.E.; Shulaeva, M.M.; Syakaev, V.V.; Semenov, V.E.; Antipin, I.S. Synthesis of 6-Methyluracilpentylviologen Resorcinarene Cavitand. Molbank 2022, 2022, M1507. https://doi.org/10.3390/M1507
Ziganshina AY, Mansurova EE, Shulaeva MM, Syakaev VV, Semenov VE, Antipin IS. Synthesis of 6-Methyluracilpentylviologen Resorcinarene Cavitand. Molbank. 2022; 2022(4):M1507. https://doi.org/10.3390/M1507
Chicago/Turabian StyleZiganshina, Albina Y., Elina E. Mansurova, Marina M. Shulaeva, Viktor V. Syakaev, Vyacheslav E. Semenov, and Igor S. Antipin. 2022. "Synthesis of 6-Methyluracilpentylviologen Resorcinarene Cavitand" Molbank 2022, no. 4: M1507. https://doi.org/10.3390/M1507
APA StyleZiganshina, A. Y., Mansurova, E. E., Shulaeva, M. M., Syakaev, V. V., Semenov, V. E., & Antipin, I. S. (2022). Synthesis of 6-Methyluracilpentylviologen Resorcinarene Cavitand. Molbank, 2022(4), M1507. https://doi.org/10.3390/M1507





