Methyl 12-Methyl-3,9-dinitro-5,6,7,12-tetrahydro-13-oxodibenzo[b.g]bicyclo[3.3.1]nonane-6-carboxylate and Related Compounds
Abstract
:1. Introduction
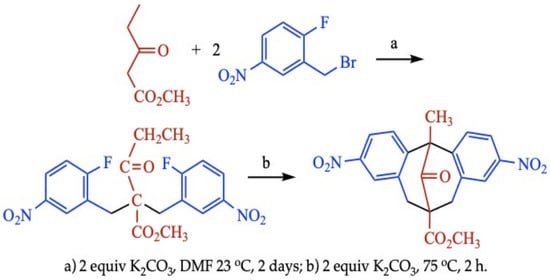
2. Results
3. Materials and Methods
3.1. General Methods
3.2. Example Procedure: Methyl 12-Methyl-3,9-dinitro-5,6,7,12-tetrahydro-13-oxodibenzo[b.g]bicyclo[3.3.1]nonane-6-carboxylate (1a)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lednicer, D. Strategies for Organic Drug Synthesis, 2nd ed.; John Wiley and Sons, Inc.: New York, NY, USA, 2008; ISBN 978-0-470-19039-5. [Google Scholar]
- Tietze, L. Domino Reactions: Concepts for Efficient Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2014; ISBN 978-3-527-33432-2. [Google Scholar]
- Nammalwar, B.; Bunce, R.A. Recent syntheses of 1,2,3,4-tetrahydroquinolines, 2,3-dihydro-4(1H)-quinolinones and 4(1H)-quinolinones using a domino strategy. Molecules 2014, 19, 204–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowley, B.M.; Mori, Y.; McComas, C.C.; Tang, D.; Boger, D.L. Total synthesis of the ristocetin aglycon. J. Am. Chem. Soc. 2004, 126, 4310–4317. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Zhu, Z.; Li, Z.; Hao, E.; Jiao, L. Novel expanded porphyrinoids with multiple-inner-ring-fusion and/or tunable aromaticity. Chin. Chem. Lett. 2019, 30, 1895–1902. [Google Scholar] [CrossRef]
- Chatterjee, T.; Srinivasan, A.; Ravikanth, M.; Chandrashekar, T.K. Smaragdyrins and sapphyrins analogues. Chem. Rev. 2017, 117, 3329–3376. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; O'Brien, H.M.; Kawasaki, H.; Takaya, H.; Nakamura, M. Ligand-free iron-catalyzed C–F amination of diarylamines: A one pot regioselective synthesis of diaryl dihydrophenazines. Org. Lett. 2019, 21, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Takeda, H.; Kuratsu, M.; Suzuki, S.; Kozaki, M.; Shiomi, D.; Sato, K.; Takui, T.; Okada, K. Radical-substituted dihydrophenazine radical cation salts: Molecular packing structure and bulk magnetic property. Pure Appl. Chem. 2010, 82, 1025–1032. [Google Scholar] [CrossRef]
- Lee, J.; Shizu, K.; Tanaka, H.; Nakanotani, H.; Yasuda, T.; Kaji, H.; Adachi, C. Controlled emission colors and singlet-triplet energy gaps of dihydrophenazine-based thermally activated delayed fluorescence emitters. J. Mater. Chem. C 2015, 3, 2175–2181. [Google Scholar] [CrossRef]
- Theriot, J.C.; Lim, C.-H.; Yang, H.; Ryan, M.D.; Musgrave, C.B.; Miyaki, G.M. Organocatalyzed atom transfer radical polymerization driven by visible light. Science 2016, 352, 1082–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunce, R.A.; Grant, M.T. 10-Alkyl-2,7-dinitro-9(10H)-acridinones by tandem SNAr reactions. Org. Prep. Proced. Int. 2011, 43, 265–275. [Google Scholar] [CrossRef]
- Bunce, R.A.; Rogers, D.; Nago, T.; Bryant, S.A. 4H-1-Benzopyrans by a tandem SN2-SNAr reaction. J. Heterocycl. Chem. 2008, 45, 547–550. [Google Scholar] [CrossRef]

| Compound 5 | Compound 1 | |||||
|---|---|---|---|---|---|---|
| Entry | R | X | Yield (%) | m.p. (°C) | Yield (%) | m.p. (°C) |
| a | CH3 | NO2 | 64 | 112–114 | 82 | 267–268 |
| b | CH2CH3 | NO2 | 77 | 83–84 | 81 | 238–239 |
| c | CH2CH2CH2CH3 | NO2 | 64 | 96–98 | 87 | 209–210 |
| d | CH2Ph | NO2 | 65 | 151–152 | 85 | 240–241 |
| e | CH3 | CN | 82 | 103–104 | 72 | 243–245 |
| f | CH2CH2CH2CH3 | CN | 65 | oil | 82 | 190–191 |
| g | CH2Ph | CN | 80 | 120–121 | 74 | 272–274 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nanney, D.R.; Bunce, R.A. Methyl 12-Methyl-3,9-dinitro-5,6,7,12-tetrahydro-13-oxodibenzo[b.g]bicyclo[3.3.1]nonane-6-carboxylate and Related Compounds. Molbank 2022, 2022, M1526. https://doi.org/10.3390/M1526
Nanney DR, Bunce RA. Methyl 12-Methyl-3,9-dinitro-5,6,7,12-tetrahydro-13-oxodibenzo[b.g]bicyclo[3.3.1]nonane-6-carboxylate and Related Compounds. Molbank. 2022; 2022(4):M1526. https://doi.org/10.3390/M1526
Chicago/Turabian StyleNanney, Dylan R., and Richard A. Bunce. 2022. "Methyl 12-Methyl-3,9-dinitro-5,6,7,12-tetrahydro-13-oxodibenzo[b.g]bicyclo[3.3.1]nonane-6-carboxylate and Related Compounds" Molbank 2022, no. 4: M1526. https://doi.org/10.3390/M1526