1-[2-(1H-Pyrrole-2-carbonyl)phenyl]-3-(4-methoxyphenyl)urea
Abstract
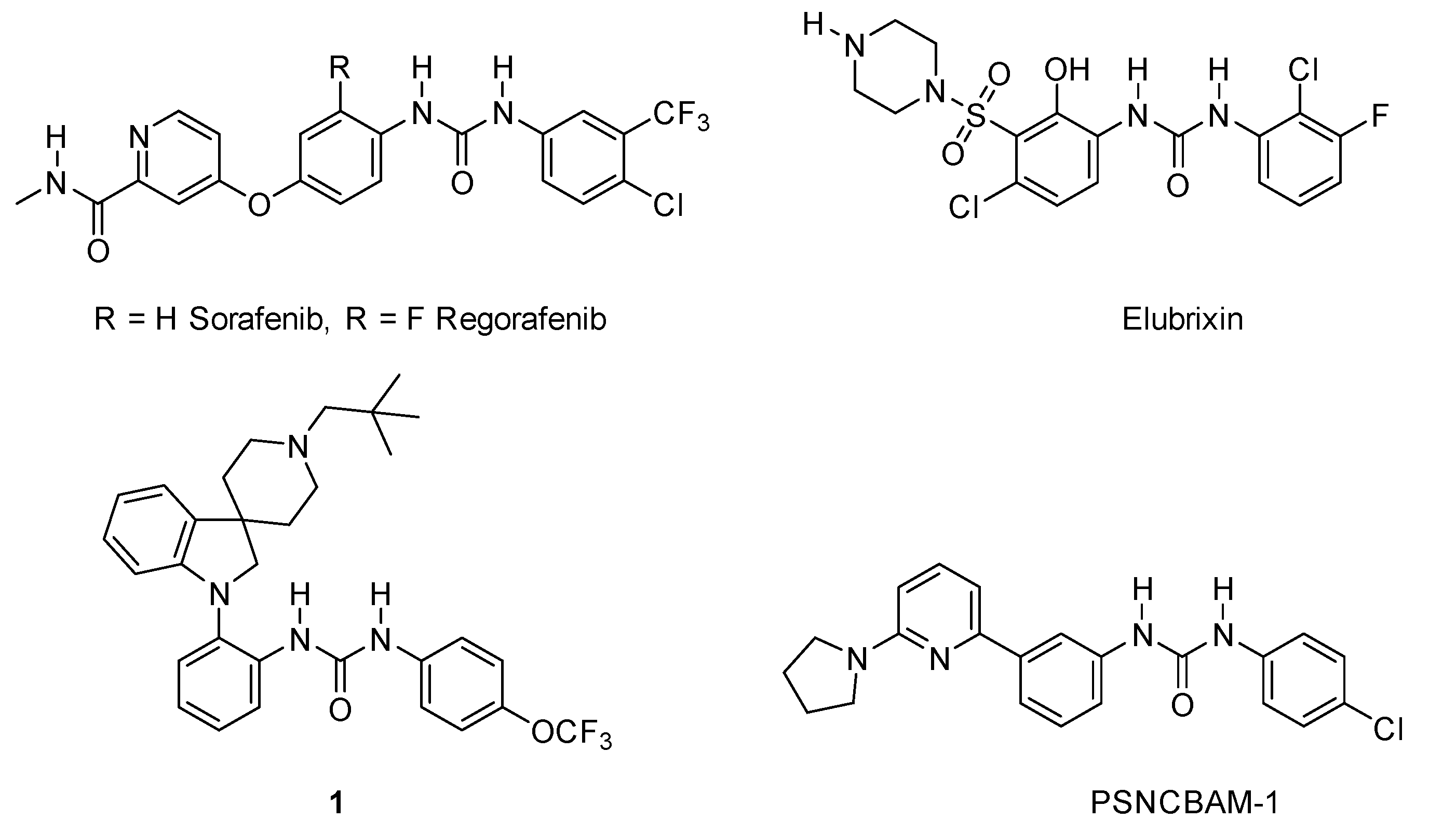
:1. Introduction
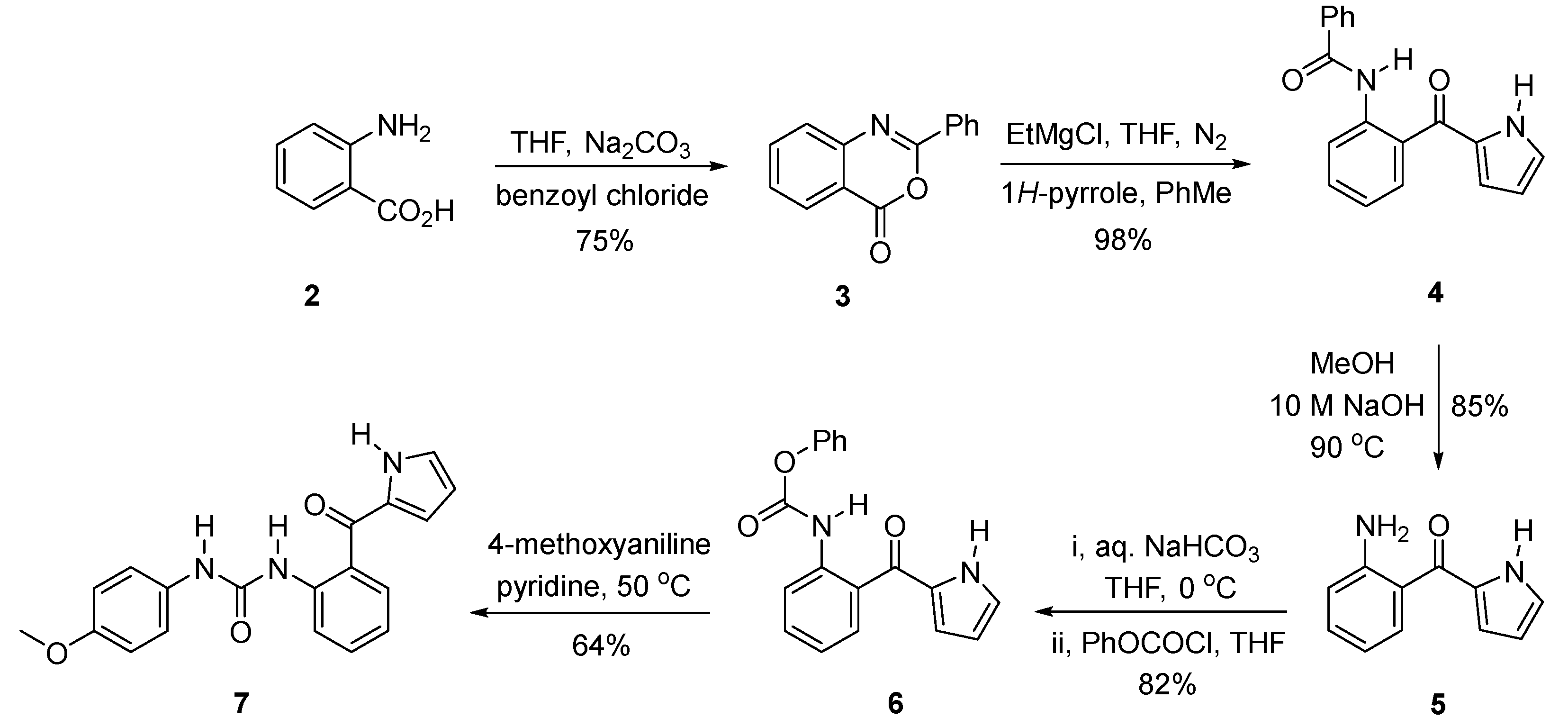
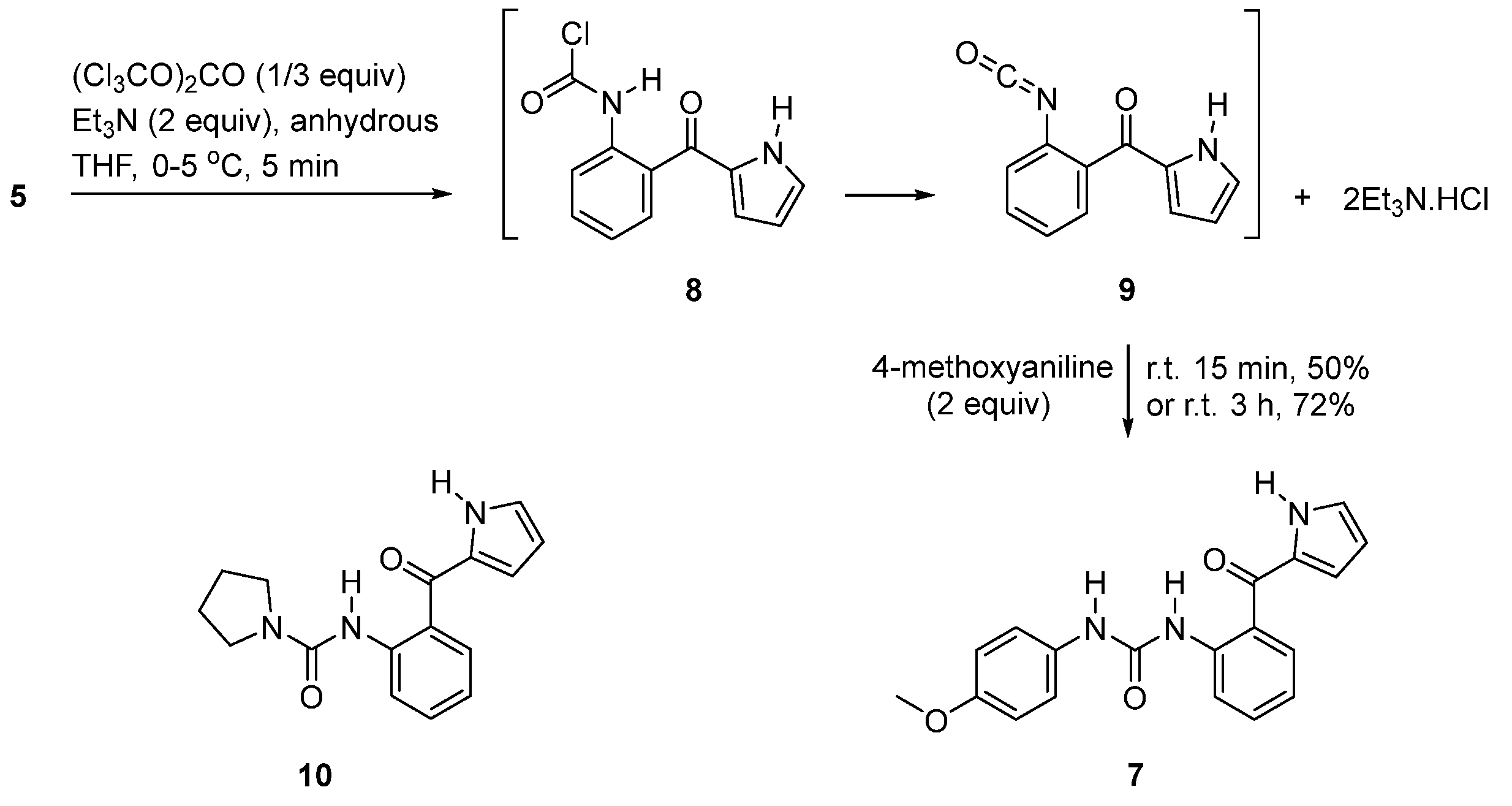
2. Results
3. Discussion
4. Materials and Methods
4.1. 2-Phenyl-4H-3,1-benzooxazin-4-one (3)
4.2. N-(2-(1H-pyrrole-2-carbonyl)phenyl)benzamide (4)
4.3. (2-Aminophenyl)(1H-pyrrol-2-yl)methanone (5)
4.4. Phenyl (2-(1H-pyrrole-2-carbonyl)phenyl)carbamate (6)
4.5. 1-(2-(1H-Pyrrole-2-carbonyl)phenyl)-3-(4-methoxyphenyl)urea (7)
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ghosh, A.K.; Brindisi, B. Urea Derivatives in Modern Drug Discovery and Medicinal Chemistry. J. Med. Chem. 2020, 63, 2751–2788. [Google Scholar] [CrossRef]
- Garuti, L.; Roberti, M.; Bottegoni, G.; Ferraro, M. Diaryl Urea: A Privileged Structure in Anticancer Agents. Curr. Med. Chem. 2016, 23, 1528–1548. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Carter, C.; Lynch, M.; Lowinger, T.; Dumas, J.; Smith, R.A.; Schwartz, B.; Simantov, R.; Kelley, S. Discovery and development of sorafenib: A multikinase inhibitor for treating cancer. Nat. Rev. Drug Discov. 2006, 5, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.H.; Shen, Y.C.; Shao, Y.Y.; Hsu, C.; Cheng, A.L. Sorafenib in advanced hepatocellular carcinoma: Current status and future perspectives. J. Hepatocell. Carcinoma 2014, 1, 85–99. [Google Scholar]
- Strumberg, D.; Scheulen, M.E.; Schultheis, B.; Richly, H.; Frost, A.; Buchert, M.; Christensen, O.; Jeffers, M.; Heinig, R.; Boix, O.; et al. Regorafenib (BAY 73-4506) in advanced colorectal cancer: A phase I study. Br. J. Cancer 2012, 106, 1722–1727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crona, D.J.; Keisler, M.D.; Walko, C.M. Regorafenib: A novel multitargeted tyrosine kinase inhibitor for colorectal cancer and gastrointestinal stromal tumors. Ann. Pharmacother. 2013, 47, 1685–1696. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Lu, H.; Xu, Z.; Luan, L.; Li, C.; Xu, Y.; Dong, K.; Zhang, J.; Li, X.; Li, Y.; et al. Discovery of CNS Penetrant CXCR2 Antagonists for the Potential Treatment of CNS Demyelinating Disorders. ACS Med. Chem. Lett. 2016, 7, 397–402. [Google Scholar] [CrossRef] [Green Version]
- Jing, L.; Qiu, Y.; Zhang, Y.; Li, J. −X. Effects of the cannabinoid CB1 receptor allosteric modulator ORG 27569 on reinstatement of cocaine- and methamphetamine-seeking behavior in rats. Drug Alcohol. Depend. 2014, 143, 251. [Google Scholar] [CrossRef] [Green Version]
- Qiao, J.X.; Wang, T.C.; Ruel, R.; Thibeault, C.; L’Heureux, A.; Schumacher, W.A.; Spronk, S.A.; Hiebert, S.; Bouthillier, G.; Lloyd, J.; et al. Conformationally Constrained ortho-Anilino Diaryl Ureas: Discovery of 1-(2-(1′-Neopentylspiro[indoline-3,4′-piperidine]-1-yl)phenyl)-3-(4-(trifluoromethoxy)phenyl)urea, a Potent, Selective, and Bioavailable P2Y1 Antagonist. J. Med. Chem. 2013, 56, 9275–9295. [Google Scholar] [CrossRef]
- Steverding, D. The development of drugs for treatment of sleeping sickness: A historical review. Parasites Vectors 2010, 3, 15. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.W.; Sarantakis, D.; Terpinski, J.; Kumar, T.R.S.; Tsai, H.-C.; Kuo, M.; Ager, A.L.; Jacobs, W.R., Jr.; Schiehser, G.A.; Ekins, S.; et al. Novel diaryl ureas with efficacy in a mouse model of malaria. Bioorg. Med. Chem. Lett. 2013, 23, 1022–1025. [Google Scholar] [CrossRef] [PubMed]
- Keche, A.P.; Hatnapure, G.D.; Tale, R.H.; Rodge, A.H.; Kamble, V.M. Synthesis, anti-inflammatory and antimicrobial evaluation of novel 1-acetyl-3,5-diaryl-4,5-dihydro(1H)pyrazole derivatives bearing urea, thiourea and sulfonamide moieties. Bioorg. Med. Chem. Lett. 2012, 22, 6611–6615. [Google Scholar] [CrossRef] [PubMed]
- Keche, A.P.; Hatnapure, G.D.; Tale, R.H.; Rodge, A.H.; Birajdar, S.S.; Kamble, V.M. A novel pyrimidine derivative with aryl urea, thiourea and sulfonamide moieties: Synthesis, anti-inflammatory and antimicrobial evaluation. Bioorg. Med. Chem. Lett. 2012, 22, 3445–3448. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.G.; Laufer, S.; Mangannavar, C.; Garlapati, A. Design, synthesis and characterization of N′,N″-diaryl ureas as p38 kinase inhibitors. Med. Chem. 2013, 9, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Velappan, A.B.; Raja, M.R.C.; Datta, D.; Tsai, Y.T.; Halloum, I.; Wan, B.; Kremer, L.; Gramajo, H.; Franzblau, S.G.; Mahapatra, S.K.; et al. Attenuation of Mycobacterium species through direct and macrophage mediated pathway by unsymmetrical diaryl urea. Eur. J. Med. Chem. 2017, 125, 825–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckert, H.; Forster, B. Triphosgene, a Crystalline Phosgene Substitute. Angew. Chem. Int. Ed. Engl. 1987, 26, 894–895. [Google Scholar] [CrossRef]
- Gallou, I. Unsymmetrical Ureas. Synthetic Methodologies and Application in Drug Design. Org. Prep. Proced. Int. 2007, 39, 355–383. [Google Scholar] [CrossRef]
- Kulkarni, A.R.; Garai, S.; Thakur, G.A. Scalable, One-Pot, Microwave-Accelerated Tandem Synthesis of Unsymmetrical Urea Derivatives. J. Org. Chem. 2017, 82, 992–999. [Google Scholar] [CrossRef]
- Mozaffari, M.; Nowrouzi, N. Palladium-Catalyzed Synthesis of Symmetrical and Unsymmetrical Ureas Using Chromium Hexacarbonyl as a Convenient and Safe Alternative Carbonyl Source. Eur. J. Org. Chem. 2019, 7541–7544. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, N.; Sharma, R.; Bhargava, G.; Mahajan, D. Direct Conversion of Carboxylic Acids to Various Nitrogen-Containing Compounds in the One-Pot Exploiting Curtius Rearrangement. J. Org. Chem. 2019, 84, 11323–11334. [Google Scholar] [CrossRef]
- Ahmed, R.; Gupta, R.; Akhter, Z.; Kumara, M.; Singh, P.P. TCT-mediated click chemistry for the synthesis of nitrogen-containing functionalities: Conversion of carboxylic acids to carbamides, carbamates, carbamothioates, amides and amines. Org. Biomol. Chem. 2022, 20, 4942–4948. [Google Scholar] [CrossRef] [PubMed]
- Wanga, L.; Wang, H.; Wang, Y.; Shen, M.; Li, S. Photocatalyzed synthesis of unsymmetrical ureas via the oxidative decarboxylation of oxamic acids with PANI-g-C3N4-TiO2 composite under visible light. Tetrahedron Lett. 2020, 61, 151962. [Google Scholar] [CrossRef]
- Yadav, A.K.; Srivastava, V.P.; Yadav, L.D.S. An easy access to unsymmetrical ureas: A photocatalytic approach to the Lossen rearrangement. RSC Adv. 2014, 4, 24498–24503. [Google Scholar] [CrossRef]
- Kotecki, B.J.; Fernando, D.P.; Haight, A.R.; Lukin, K.A. A General Method for the Synthesis of Unsymmetrically Substituted Ureas via Palladium-Catalyzed Amidation. Org. Lett. 2009, 11, 947–950. [Google Scholar] [CrossRef] [PubMed]
- Breitler, S.; Oldenhuis, N.J.; Fors, B.P.; Buchwald, S.L. Synthesis of Unsymmetrical Diarylureas via Pd-Catalyzed C−N Cross-Coupling Reactions. Org. Lett. 2011, 13, 3262–3265. [Google Scholar] [CrossRef] [Green Version]
- Vinogradova, E.V.; Fors, B.P.; Buchwald, S.L. Palladium-Catalyzed Cross-Coupling of Aryl Chlorides and Triflates with Sodium Cyanate: A Practical Synthesis of Unsymmetrical Ureas. J. Am. Chem. Soc. 2012, 134, 11132–11135. [Google Scholar] [CrossRef] [Green Version]
- Shamanth, S.; Chaithra, N.; Gurukiran, M.; Mamatha, M.; Lokanath, N.K.; Rangappa, K.S.; Mantelingu, K. I2-Catalyzed transformation of o-aminobenzamide to o-ureidobenzonitrile using isothiocyanates. Org. Biomol. Chem. 2020, 18, 2678–2684. [Google Scholar] [CrossRef]
- Rotas, G.; Kimbaris, A.; Varvounis, G. Synthesis of a novel pyrrolo [1,2-c][1.3]benzodiazepine analogue of VPA-985. Tetrahedron 2011, 67, 7805–7810. [Google Scholar] [CrossRef]
- Rotas, G.; Varvounis, G. Tunable Reactivity of Triphosgene-Triethylamine: A study on the synthesis of pyrrole-based tricyclics. Tetrahedron 2020, 76, 131527. [Google Scholar] [CrossRef]
- Papadaki, S.; Moschonas, I.; Peroulis, M.; Tatsidou, P.; Sarantou, A.; Tellis, C.; Varvounis, G.; Tselepis, A. Investigation of the Antithrombotic and Antiplatelet Effect of a New Urea Analog, on Carotid Artery Thrombosis in Rabbits. Thromb. Res. 2016, 141 (Suppl. 1), S2. [Google Scholar]
- Qurban, J.; Elsherbini, M.; Alharbi, H.; Wirth, T. Synthesis, characterisation, and reactivity of novel pseudocyclic hypervalent iodine reagents with heteroaryl carbonyl substituents. Chem. Commun. 2019, 55, 7998–8000. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarantou, A.G.; Varvounis, G. 1-[2-(1H-Pyrrole-2-carbonyl)phenyl]-3-(4-methoxyphenyl)urea. Molbank 2023, 2023, M1531. https://doi.org/10.3390/M1531
Sarantou AG, Varvounis G. 1-[2-(1H-Pyrrole-2-carbonyl)phenyl]-3-(4-methoxyphenyl)urea. Molbank. 2023; 2023(1):M1531. https://doi.org/10.3390/M1531
Chicago/Turabian StyleSarantou, Antonia G., and George Varvounis. 2023. "1-[2-(1H-Pyrrole-2-carbonyl)phenyl]-3-(4-methoxyphenyl)urea" Molbank 2023, no. 1: M1531. https://doi.org/10.3390/M1531





