Tetra(phenylethynyl)tin Is a New Reagent for Solvent-Free Alkynylation of Imines
Abstract
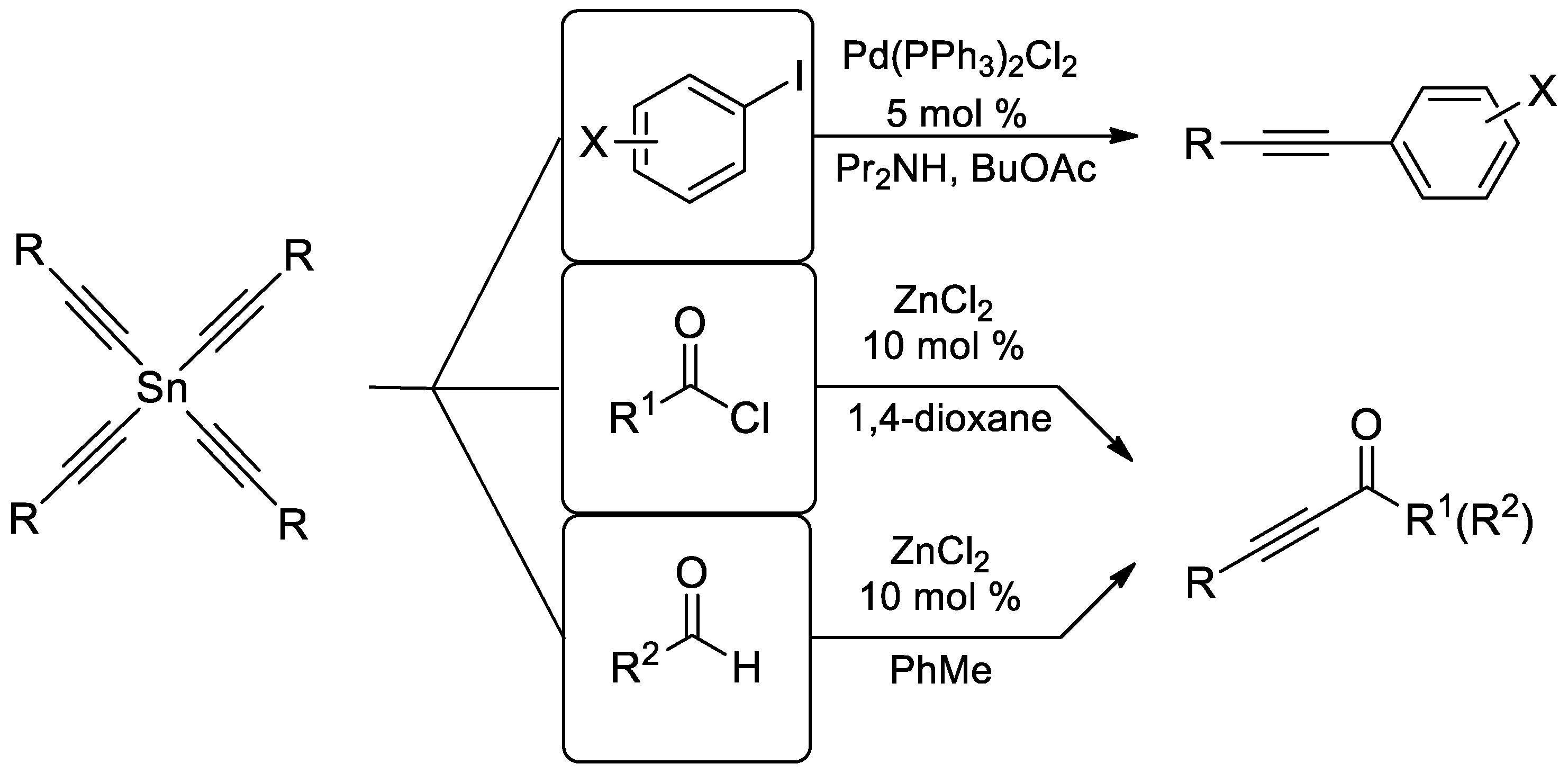
:1. Introduction
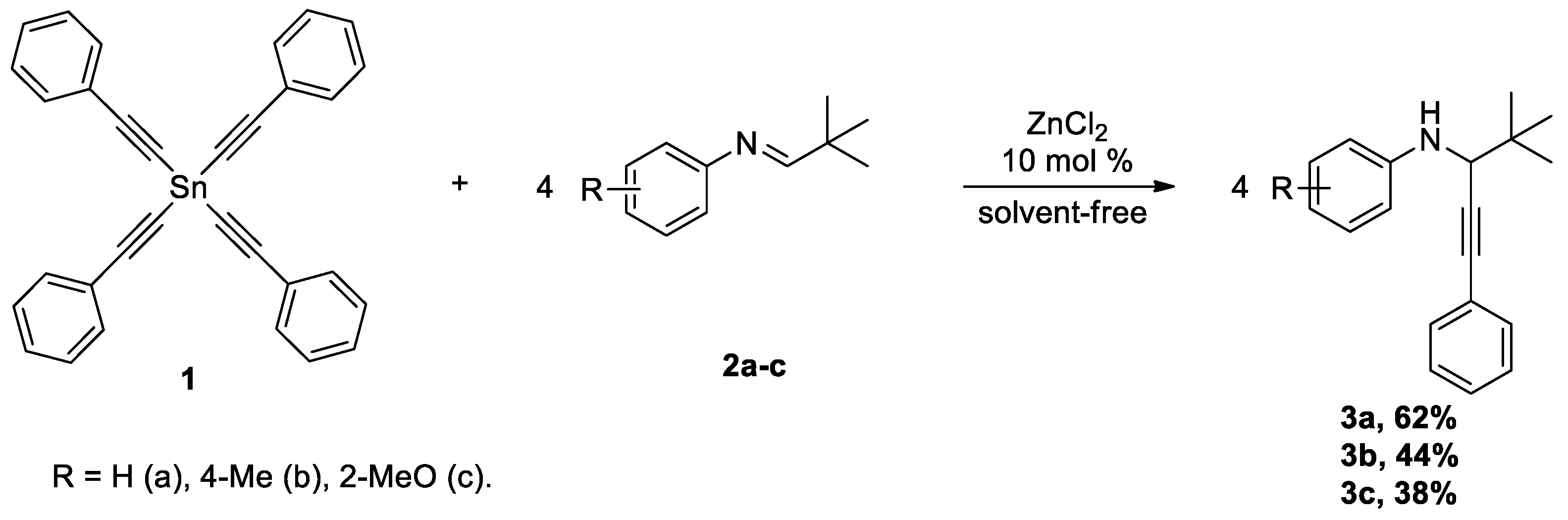
2. Results and Discussion
3. Materials and Methods
General Procedure for the Synthesis of Propargylamine
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diederich, F.; Stang, P.J.; Tykwinski, R.R. Acetylene Chemistry: Chemistry, Biology, and Material Science; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; pp. 1–508. [Google Scholar] [CrossRef]
- Trost, B.M.; Li, C.-J. Modern Alkyne Chemistry: Catalytic and Atom-Economic Transformations; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; pp. 1–402. [Google Scholar] [CrossRef]
- Rodygin, K.S.; Werner, G.; Kucherov, F.A.; Ananikov, V.P. Calcium carbide: A unique reagent for organic synthesis and nanotechnology. Chem. Asian J. 2016, 11, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Rodygin, K.S.; Ledovskaya, M.S.; Voronin, V.V.; Lotsman, K.A.; Ananikov, V.P. Calcium carbide: Versatile synthetic applications, green methodology and sustainability. Eur. J. Org. Chem. 2021, 2021, 43–52. [Google Scholar] [CrossRef]
- Van Bonn, P.; Bolm, C. Mechanochemical synthesis of diarylethynes from aryl iodides and CaC2. Synlett 2022, 33, 893–897. [Google Scholar] [CrossRef]
- Nandy, S.; Paul, S.; Das, K.K.; Kumar, P.; Ghorai, D.; Panda, S. Synthesis and reactivity of alkynyl boron compounds. Org. Biomol. Chem. 2021, 19, 7276–7297. [Google Scholar] [CrossRef] [PubMed]
- Tani, T.; Sawatsugawa, Y.; Sano, Y.; Hirataka, Y.; Takahashi, N.; Hashimoto, S.; Sugiura, T.; Tsuchimoto, T. Alkynyl−B(dan)s in Various Palladium-Catalyzed Carbon−Carbon Bond-Forming Reactions Leading to Internal Alkynes, 1,4-Enynes, Ynones, and Multiply Substituted Alkenes. Adv. Synth. Catal. 2019, 361, 1815–1834. [Google Scholar] [CrossRef]
- Stefani, H.A.; Cella, R.; Vieira, A.S. Recent advances in organotrifluoroborates chemistry. Tetrahedron 2007, 63, 3623–3658. [Google Scholar] [CrossRef]
- Buendia, M.B.; Balin, J.-G.J.; Andersen, M.E.; Lian, Z.; Kramer, S. Copper-catalyzed alkynylation of benzylic C-H bonds with alkynylboronic esters. Synlett 2022, 33, 150–154. [Google Scholar] [CrossRef]
- Micouin, L.; Piccardi, R.; Turcaud, S.; Benedetti, E. Synthesis and Reactivity of Mixed Dimethylalkynylaluminum Reagents. Synthesis 2019, 50, 97–106. [Google Scholar] [CrossRef]
- Kafuta, K.; Rugen, C.J.; Heilmann, T.; Liu, T.; Golz, C.; Alcarazo, M. Reactivity of 5-(Alkynyl)dibenzothiophenium Salts: Synthesis of Diynes, Vinyl Sulfones, and Phenanthrenes. Eur. J. Org. Chem. 2021, 51, 4038–4048. [Google Scholar] [CrossRef]
- Waldecker, B.; Kraft, F.; Golz, C.; Alcarazo, M. 5-(alkynyl)dibenzothiophenium triflates: Sulfur-based reagents for electrophilic alkynylation. Angew. Chem. Int. Ed. 2018, 57, 12538–12542. [Google Scholar] [CrossRef]
- Amos, S.G.E.; Cavalli, D.; Le Vaillant, F.; Waser, J. Direct photoexcitation of ethynylbenziodoxolones: An alternative to photocatalysis for alkynylation reactions. Angew. Chem. Int. Ed. 2021, 60, 23827–23834. [Google Scholar] [CrossRef]
- Amos, S.G.E.; Waser, J. Radical alkynylations with EthynylBenziodoXolones: From photocatalysis to direct excitation. Chimia 2022, 76, 312–315. [Google Scholar] [CrossRef]
- García Ruano, J.L.; Alemán, J.; Parra, A.; Marzo, L. Sulfonyl Acetylenes as Alkynylating Reagents Under Radical or Anionic Conditions. Eur. J. Org. Chem. 2014, 2014, 1577–1588. [Google Scholar] [CrossRef]
- Andrei, M.; Undheim, K. Clarithromycin macrolides modified by unsaturation at the C10-position. Phytochem. Lett. 2022, 50, 128–133. [Google Scholar] [CrossRef]
- Makhloutah, A.; Hatych, D.; Chartier, T.; Rocard, L.; Goujon, A.; Felpin, F.-X.; Hudhomme, P. An investigation of palladium-catalyzed Stille-type cross-coupling of nitroarenes in perylenediimide series. Org. Biomol. Chem. 2022, 20, 362–365. [Google Scholar] [CrossRef]
- Yokose, D.; Nagashima, Y.; Kinoshita, S.; Nogami, J.; Tanaka, K. Enantioselective synthesis of axially chiral styrene-carboxylic esters by rhodium-catalyzed chelation-controlled [2+2+2] cycloaddition. Angew. Chem. Int. Ed. 2022, 61, e202202542. [Google Scholar] [CrossRef]
- Ouadoudi, O.; Kaehler, T.; Çevik, E.G.; Bolte, M.; Stöger, B.; Virovets, A.; Lerner, H.-W.; Wagner, M. Late-stage derivatization of a (B,O)2-doped perylene. Dalton Trans. 2022, 51, 13195–13198. [Google Scholar] [CrossRef]
- Lapkin, A.; Constable, D.J.C. Green Chemistry Metrics: Measuring and Monitoring Sustainable Processes. Green Chemistry Metrics: Measuring and Monitoring Sustainable Processes; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2009; pp. 1–324. [Google Scholar] [CrossRef]
- Levashov, A.S.; Andreev, A.A.; Konshin, V.V. Lewis acid promoted direct synthesis of tetraalkynylstannanes. Tetrahedron Lett. 2015, 56, 1870–1872. [Google Scholar] [CrossRef]
- Levashov, A.S.; Buryi, D.S.; Goncharova, O.V.; Konshin, V.V.; Dotsenko, V.V.; Andreev, A.A. Tetraalkynylstannanes in the stille cross coupling reaction: A new effective approach to arylalkynes. New J. Chem. 2017, 41, 2910–2918. [Google Scholar] [CrossRef] [Green Version]
- Levashov, A.S.; Aksenov, N.A.; Aksenova, I.V.; Konshin, V.V. Oxidative coupling of tetraalkynyltin with aldehydes leading to alkynyl ketones. New J. Chem. 2017, 41, 8297–8304. [Google Scholar] [CrossRef] [Green Version]
- Levashov, A.S.; Buryi, D.S. Lewis acid promoted reaction of tetraalkynylstannanes with acyl chlorides: An effective approach towards alkynyl ketones. Tetrahedron Lett. 2017, 58, 4476–4478. [Google Scholar] [CrossRef]
- Lauder, K.; Toscani, A.; Scalacci, N.; Castagnolo, D. Synthesis and reactivity of propargylamines in organic chemistry. Chem. Rev. 2017, 117, 14091–14200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jesin, I.; Nandi, G.C. Recent advances in the A3 coupling reactions and their applications. Eur. J. Org. Chem. 2019, 2019, 2704–2720. [Google Scholar] [CrossRef]
- Volkova, Y.; Baranin, S.; Zavarzin, I. A3 coupling reaction in the synthesis of heterocyclic compounds. Adv. Synth. Catal. 2021, 363, 40–61. [Google Scholar] [CrossRef]
- Monleón, A.; Blay, G.; Pedro, J.R. Catalytic enantioselective cyclopropylalkynylation of aldimines generated in situ from α-amido sulfones. Molecules 2022, 27, 3763. [Google Scholar] [CrossRef]
- Blay, G.; Monleon, A.; Pedro, J. Recent Developments in Asymmetric Alkynylation of Imines. Curr. Org. Chem. 2009, 13, 1498–1539. [Google Scholar] [CrossRef]
- Jiang, B.; Si, Y.-G. Lewis acid promoted alkynylation of imines with terminal alkynes: Simple, mild and efficient preparation of propargylic amines. Tetrahedron Lett. 2003, 44, 6767–6768. [Google Scholar] [CrossRef]
- Mokuolu, Q.F.; Duckmanton, P.A.; Hitchcock, P.B.; Wilson, C.; Blake, A.J.; Shukla, L.; Love, J.B. Early-late, mixed-metal compounds supported by amidophosphine ligands. Dalton Transactions 2004, 13, 1960–1970. [Google Scholar] [CrossRef]
- Nanni, D.; Pareschi, P.; Walton, J.C. An electron paramagnetic resonance study of intermediates generated from aromatic aldimines. J. Chem. Soc. Perkin Trans. 2002, 6, 1098–1104. [Google Scholar] [CrossRef]
- Yamashita, Y.; Noguchi, A.; Fushimi, S.; Hatanaka, M.; Kobayashi, S. Chiral metal salts as ligands for catalytic asymmetric mannich reactions with simple amides. J. Am. Chem. Soc. 2021, 143, 5598–5604. [Google Scholar] [CrossRef]
- Spesivaya, E.S.; Lupanova, I.A.; Konshina, D.N.; Konshin, V.V. Zn(OTf)2/i-Pr2NEt promoted synthesis of tetraalkynylsilanes. Tetrahedron Lett. 2021, 63, 152713. [Google Scholar] [CrossRef]
- Andreev, A.A.; Konshin, V.V.; Vinokurov, N.A.; Komarov, N.V. Synthesis of tri-and tetraalkynylgermanes. Russ. Chem. Bull. 2006, 55, 1430–1432. [Google Scholar] [CrossRef]
- Li, C.-J.; Wei, C. Highly efficient Grignard-type imine additions via C-H activation in water and under solvent-free conditions. Chem. Commun. 2002, 3, 268–269. [Google Scholar] [CrossRef]
- Zani, L.; Alesi, S.; Cozzi, P.G.; Bolm, C. Dimethylzinc-Mediated Alkynylation of Imines. J. Org. Chem. 2006, 71, 1558–1562. [Google Scholar] [CrossRef]


| Entry | Lewis Acid (10 mol %) | Solvent | Temp, °C | Time, h | Yield 3a,% (GS/MS) |
|---|---|---|---|---|---|
| 1 | ZnCl2 | PhMe | 100 | 3 | 36 |
| 2 | ZnCl2 | PhMe | 100 | 9 | 92 (7 **) |
| 3 | InCl3 | PhMe | 100 | 9 | 58 |
| 4 | AlCl3 | PhMe | 100 | 9 | 17 |
| 5 | BF3·OEt2 | PhMe | 100 | 9 | 52 |
| 6 | ZnCl2 | DCE | 80 | 9 | 30 |
| 7 | ZnCl2 | DCM | 30 | 9 | 3 |
| 8 | ZnCl2 | 1,4-dioxane | 100 | 9 | - |
| 9 | ZnCl2 | - | 100 | 12 | 98 |
| 10 | InBr3 | - | 100 | 9 | 25 |
| 11 | Sc(OTf)3 | - | 100 | 9 | 9 |
| 12 | Cu(OTf)2 | - | 100 | 9 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levashov, A.S.; Dvirnaya, E.V.; Konshina, D.N.; Konshin, V.V. Tetra(phenylethynyl)tin Is a New Reagent for Solvent-Free Alkynylation of Imines. Molbank 2023, 2023, M1534. https://doi.org/10.3390/M1534
Levashov AS, Dvirnaya EV, Konshina DN, Konshin VV. Tetra(phenylethynyl)tin Is a New Reagent for Solvent-Free Alkynylation of Imines. Molbank. 2023; 2023(1):M1534. https://doi.org/10.3390/M1534
Chicago/Turabian StyleLevashov, Andrey S., Elena V. Dvirnaya, Dzhamilay N. Konshina, and Valery V. Konshin. 2023. "Tetra(phenylethynyl)tin Is a New Reagent for Solvent-Free Alkynylation of Imines" Molbank 2023, no. 1: M1534. https://doi.org/10.3390/M1534
APA StyleLevashov, A. S., Dvirnaya, E. V., Konshina, D. N., & Konshin, V. V. (2023). Tetra(phenylethynyl)tin Is a New Reagent for Solvent-Free Alkynylation of Imines. Molbank, 2023(1), M1534. https://doi.org/10.3390/M1534








