2,7-Bis(pyridin-3-ylethynyl)fluoren-9-one
Abstract
1. Introduction
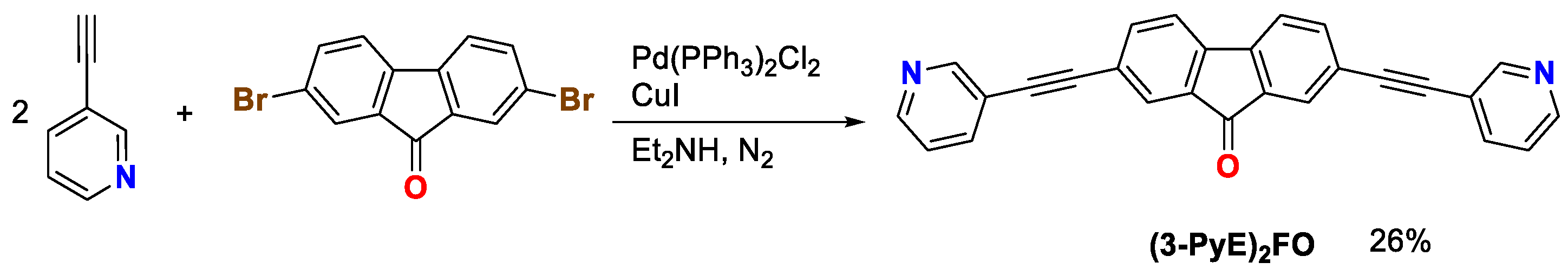
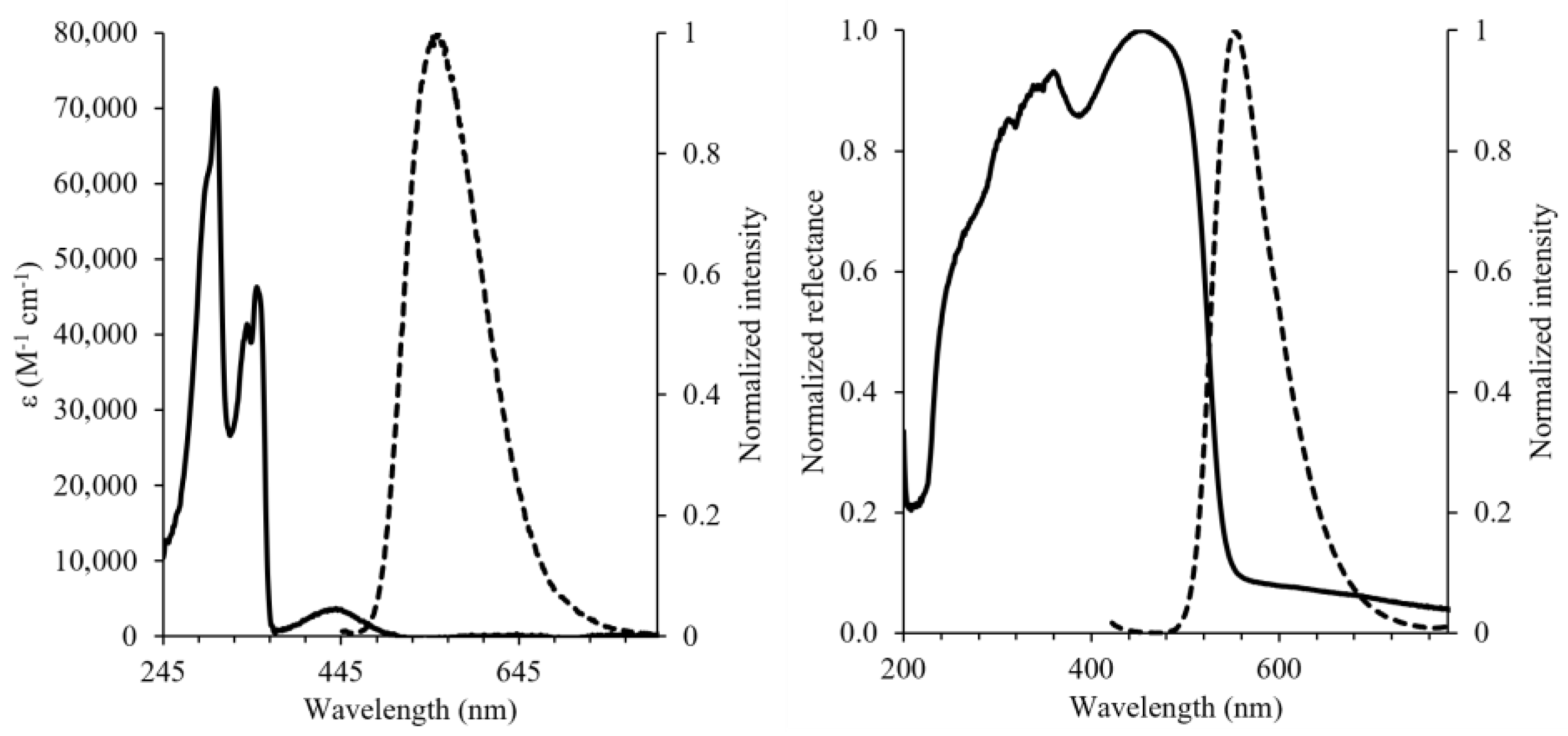
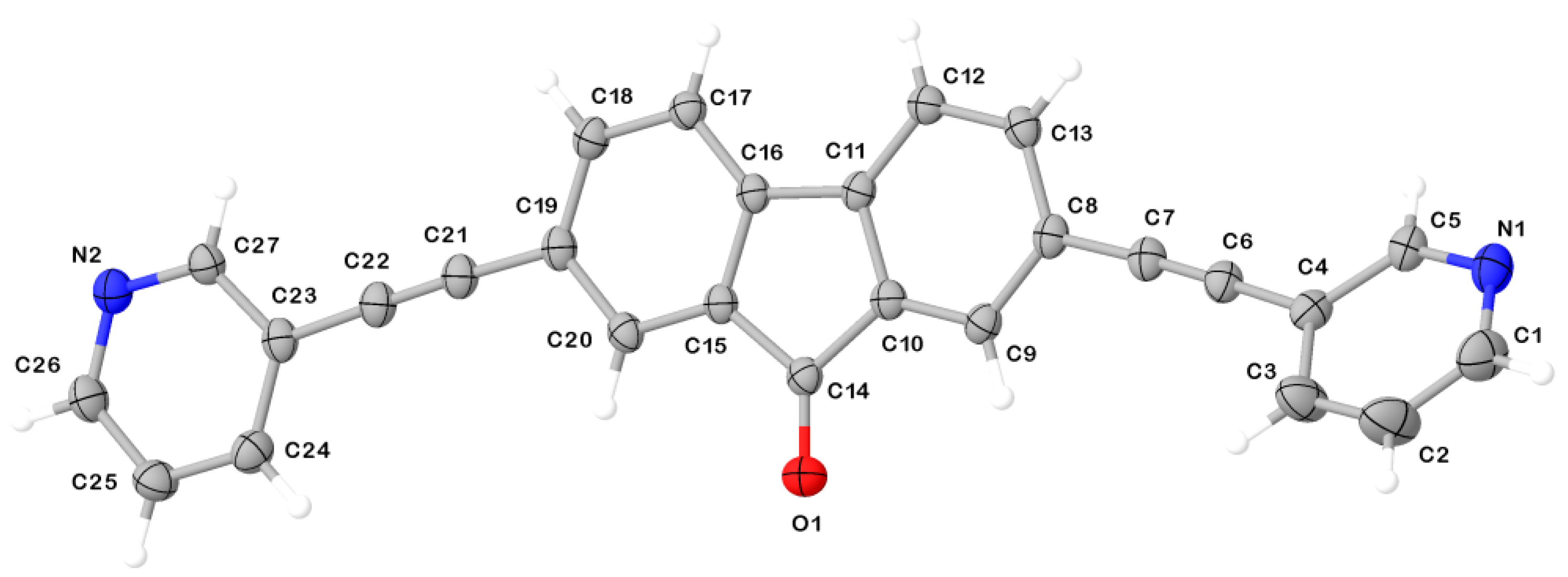
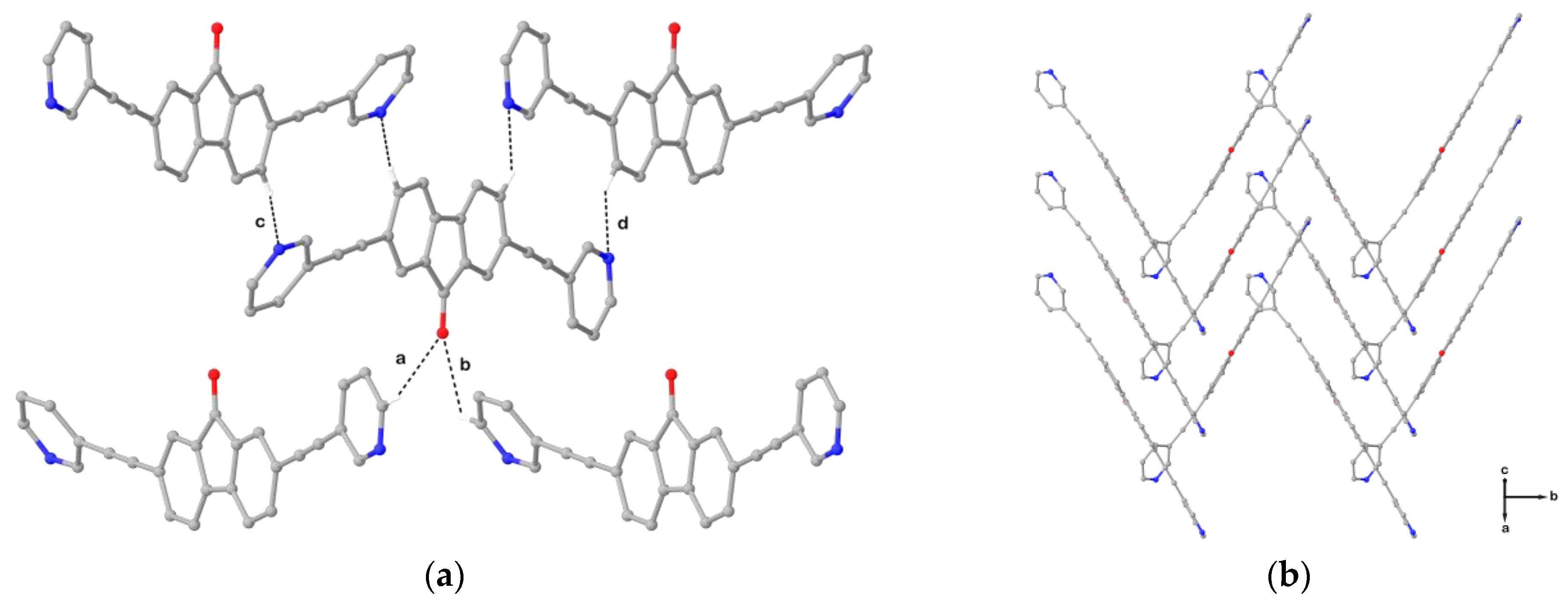
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Synthesis of 2,7-Bis(pyridin-3-ylethynyl)fluoren-9-one [(3-PyE)2FO]
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albano, V.G.; Aragoni, M.C.; Arca, M.; Castellari, C.; Demartin, F.; Devillanova, F.A.; Isaia, F.; Lippolis, V.; Loddo, L.; Verani, G. An unprecedented example of a cis-phosphonodithioato nickel(ii) complex built by an extensive hydrogen bonding supramolecular network. Chem. Commun. 2002, 11, 1170–1171. [Google Scholar] [CrossRef] [PubMed]
- Semin, S.; Li, X.; Duan, Y.; Rasing, T.; Semin, S.; Li, X.; Duan, Y.; Rasing, T. Nonlinear Optical Properties and Applications of Fluorenone Molecular Materials. Adv. Opt. Mater. 2021, 9, 2100327. [Google Scholar] [CrossRef]
- Liu, B.; Yu, W.L.; Lai, Y.H.; Huang, W. Blue-Light-Emitting Fluorene-Based Polymers with Tunable Electronic Properties. Chem. Mater. 2001, 13, 1984–1991. [Google Scholar] [CrossRef]
- Gong, X.; Iyer, P.K.; Moses, D.; Bazan, G.C.; Heeger, A.J.; Xiao, S.S. Stabilized Blue Emission from Polyfluorene-Based Light-Emitting Diodes: Elimination of Fluorenone Defects. Adv. Funct. Mater. 2003, 13, 325–330. [Google Scholar] [CrossRef]
- Guo, H.D.; Guo, X.M.; Batten, S.R.; Song, J.F.; Song, S.Y.; Dang, S.; Zheng, G.L.; Tang, J.K.; Zhang, H.J. Hydrothermal Synthesis, Structures, and Luminescent Properties of Seven d10 Metal- Organic Frameworks Based on 9,9-Dipropylfluorene-2,7- Dicarboxylic Acid (H2DFDA). Cryst. Growth Des. 2009, 9, 1394–1401. [Google Scholar] [CrossRef]
- Tessarolo, J.; Lee, H.; Sakuda, E.; Umakoshi, K.; Clever, G.H. Integrative Assembly of Heteroleptic Tetrahedra Controlled by Backbone Steric Bulk. J. Am. Chem. Soc. 2021, 143, 6339–6344. [Google Scholar] [CrossRef]
- Moreau, F.; Audebrand, N.; Poriel, C. 9,9′-Spirobifluorene Based Zinc Coordination Polymers: A Study on Linker Geometry and Topology. CrystEngComm 2019, 22, 293–303. [Google Scholar] [CrossRef]
- Podda, E.; Arca, M.; Pintus, A.; Lippolis, V.; Caltagirone, C.; Coles, S.J.; Orton, J.B.; Ennas, G.; Picci, G.; Davies, R.P.; et al. On the role of torsional dynamics in the solid-state fluorescent properties of a new bifluorene-tetracarboxylic acid and its supramolecular assemblies: A structural and DFT investigation. CrystEngComm 2023, submitted.
- Xu, J.; Semin, S.; Niedzialek, D.; Kouwer, P.H.; Fron, E.; Coutino, E.; Savoini, M.; Li, Y.; Hofkens, J.; Uji-I, H.; et al. Self-Assembled Organic Microfibers for Nonlinear Optics. Adv. Mater. 2013, 25, 2084–2089. [Google Scholar] [CrossRef]
- Duan, Y.; Ju, C.; Yang, G.; Fron, E.; Coutino-Gonzalez, E.; Semin, S.; Fan, C.; Balok, R.S.; Cremers, J.; Tinnemans, P.; et al. Aggregation Induced Enhancement of Linear and Nonlinear Optical Emission from a Hexaphenylene Derivative. Adv. Funct. Mater. 2016, 26, 8968–8977. [Google Scholar] [CrossRef]
- Li, X.; Semin, S.; Estrada, L.A.; Yuan, C.; Duan, Y.; Cremers, J.; Tinnemans, P.; Kouwer, P.; Rowan, A.E.; Rasing, T.; et al. Strong Optical Nonlinearities of Self-Assembled Polymorphic Microstructures of Phenylethynyl Functionalized Fluorenones. Chin. Chem. Lett. 2018, 29, 297–300. [Google Scholar] [CrossRef]
- See Refcodes: AXOVEP, AXOVIT, COJMIZ, HACNIL, LINZUG, MACHIJ, NOMLIM, PEKCOA, SOQSAU, TOHQUE, TOWNIE, UQIVIB, ZUFZIJ. Crystal Structures Can be Accessed/Downloaded for Free from. Available online: https://www.ccdc.cam.ac.uk/structures/ (accessed on 1 January 2023).
- Wang, J.; Cheng, Y.; Zhou, J.; Tang, W. A Donor-Acceptor Liganded Metal-Organic Framework Showcases the Hydrogen-Bond-Enhanced Sensing of N-Heterocyclic Explosives. J. Mater. Chem. C Mater. 2021, 9, 12086–12093. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, M.; Chen, K.; Liu, B.; Wang, D.; You, Y.; Zhou, X.; Huang, W. Syntheses, Structures, and Properties of Four Coordination Polymers Based on 2,7-Di(Pyridin-4-yl)-9H-Fluoren-9-One. Appl. Organomet Chem. 2020, 34, e5477. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, Z.K.; Lang, F.F.; Yu, H.M.; Cao, C.; Ni, C.Y.; Wang, M.Y.; Song, Y.L.; Lang, J.P. Construction of Cluster-Based Supramolecular Wire and Rectangle. Dalton Trans. 2022, 51, 6358–6365. [Google Scholar] [CrossRef]
- Behrendt, A.; Couchman, S.M.; Jeffery, J.C.; McCleverty, J.A.; Ward, M.D. Complexes Containing Redox-Active Fluorenone-Based Ligands Linked to Redox-Active Tris(Pyrazolyl)Boratomolybdenum Fragments: Assignment of Ligand-Centred and Metal-Centred Redox Processes by EPR and UV/VIS/NIR Spectroelectrochemistry†. J. Chem. Soc. Dalton Trans. 1999, 24, 4349–4355. [Google Scholar] [CrossRef]
- Podda, E.; Coles, S.J.; Horton, P.N.; Lickiss, P.D.; Bull, O.S.; Orton, J.B.; Pintus, A.; Pugh, D.; Carla Aragoni, M.; Davies, R.P. First Example of Solid-State Luminescent Borasiloxane-Based Chiral Helices Assembled through N–B Bonds. Dalton Trans. 2021, 50, 3782–3785. [Google Scholar] [CrossRef]
- Tykwinski, R.R. Evolution in the Palladium-Catalyzed Cross-Coupling of sp- and sp2-Hybridized Carbon Atoms. Angew. Chem. Int. Ed. 2003, 42, 1566–1568. [Google Scholar] [CrossRef]
- Wood, P.A.; Olsson, T.S.G.; Cole, J.C.; Cottrell, S.J.; Feeder, N.; Galek, P.T.A.; Groom, C.R.; Pidcock, E. Evaluation of Molecular Crystal Structures Using Full Interaction Maps. CrystEngComm 2012, 15, 65–72. [Google Scholar] [CrossRef]
- Kubin, R.F.; Fletcher, A.N. Fluorescence quantum yields of some rhodamine dyes. J. Lumin. 1982, 27, 455–462. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta. Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta. Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podda, E.; Arca, M.; Pintus, A.; Demontis, V.; Lippolis, V.; Ferino, G.; Orton, J.B.; Coles, S.J.; Aragoni, M.C. 2,7-Bis(pyridin-3-ylethynyl)fluoren-9-one. Molbank 2023, 2023, M1540. https://doi.org/10.3390/M1540
Podda E, Arca M, Pintus A, Demontis V, Lippolis V, Ferino G, Orton JB, Coles SJ, Aragoni MC. 2,7-Bis(pyridin-3-ylethynyl)fluoren-9-one. Molbank. 2023; 2023(1):M1540. https://doi.org/10.3390/M1540
Chicago/Turabian StylePodda, Enrico, Massimiliano Arca, Anna Pintus, Valentina Demontis, Vito Lippolis, Giulio Ferino, James B. Orton, Simon J. Coles, and Maria Carla Aragoni. 2023. "2,7-Bis(pyridin-3-ylethynyl)fluoren-9-one" Molbank 2023, no. 1: M1540. https://doi.org/10.3390/M1540
APA StylePodda, E., Arca, M., Pintus, A., Demontis, V., Lippolis, V., Ferino, G., Orton, J. B., Coles, S. J., & Aragoni, M. C. (2023). 2,7-Bis(pyridin-3-ylethynyl)fluoren-9-one. Molbank, 2023(1), M1540. https://doi.org/10.3390/M1540








