(4-(Adamantan-1-yl)-1-(isopropyl)-1H-imidazol-2-yl)methanol
Abstract
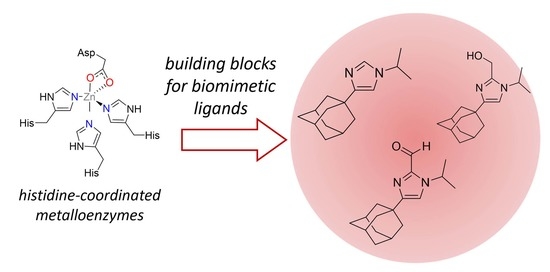
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Synthesis
3.2. Synthesis of 1-(Adamantan-1-yl)-2-bromoethanone (1)
3.3. Synthesis of 1-(Adamantan-1-yl)-2-(isopropylamino)ethanone (2)
3.4. Synthesis of 4-(Adamantan-1-yl)-1-isopropylimidazole (3)
3.5. Synthesis of 4-(Adamantan-1-yl)-1-isopropylimidazole-2-carbaldehyde (4)
3.6. Synthesis of (4-(Adamantan-1-yl)-1-isopropylimidazol-2-yl) methanol (5)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zygiel, E.M.; Nolan, E.M. Transition Metal Sequestration by the Host-Defense Protein Calprotectin. Annu. Rev. Biochem. 2018, 87, 621–643. [Google Scholar] [CrossRef] [PubMed]
- Gilston, B.A.; Skaar, E.P.; Chazin, W.J. Binding of Transition Metals to S100 Proteins. Sci. China Life Sci. 2016, 59, 792–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birukou, I.; Schweers, R.L.; Olson, J.S. Distal Histidine Stabilizes Bound O2 and Acts as a Gate for Ligand Entry in Both Subunits of Adult Human Hemoglobin*. J. Biol. Chem. 2010, 285, 8840–8854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, R.; Jones, J.C.; Lipscomb, J.D. Soluble Methane Monooxygenase. Annu. Rev. Biochem. 2019, 88, 409–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, Y.-H.; Canary, J.W. Stability and Acidity Constants for Ternary Ligand-Zinc-Hydroxo Complexes of Tetradentate Tripodal Ligands. Inorg. Chem. 2003, 42, 5107–5116. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.S.; Zamkanei, M.; Cocho, J.L. Tris(Imidazole)-Containing Phosphine: M2+ Complexes as Biomimetic Catalysts. Importance of a L:M2+-OH− in the Catalyzed Bimolecular Hydrolysis of p-Nitrophenyl Picolinate. J. Am. Chem. Soc. 1984, 106, 5222–5228. [Google Scholar] [CrossRef]
- Kimura, E.; Shionoya, M.; Mita, T.; Litaka, Y. A New Cyclam with an Appended Imidazole. The First Biomimetic Ligation of Imidazole for Axial π-Interaction with Metal Ions. J. Chem. Soc. Chem. Commun. 1987, 22, 1712–1714. [Google Scholar] [CrossRef]
- Kunz, P.C.; Reiß, G.J.; Frank, W.; Kläui, W. A Novel Water-Soluble Tripodal Imidazolyl Ligand as a Model for the Tris(Histidine) Motif of Zinc Enzymes: Nickel, Cobalt and Zinc Complexes and a Comparison with Metal Binding in Carbonic Anhydrase. Eur. J. Inorg. Chem. 2003, 2003, 3945–3951. [Google Scholar] [CrossRef]
- Pravst, I.; Zupan, M.; Stavber, S. Directed Regioselectivity of Bromination of Ketones with NBS: Solvent-Free Conditions versus Water. Tetrahedron Lett. 2006, 47, 4707–4710. [Google Scholar] [CrossRef]
- Kumar, C.S.C.; Kwong, H.C.; Mah, S.H.; Chia, T.S.; Loh, W.-S.; Quah, C.K.; Lim, G.K.; Chandraju, S.; Fun, H.-K. Synthesis and Crystallographic Insight into the Structural Aspects of Some Novel Adamantane-Based Ester Derivatives. Molecules 2015, 20, 18827–18846. [Google Scholar] [CrossRef] [PubMed]
- Moiseev, I.K.; Kalinina, M.I.; Zemtsova, M.N.; Trakhtenberg, P.L. Synthesis and Properties of Dioximes of the Adamantane Series. Zhurnal Org. Khimii 1986, 22, 2292–2296. [Google Scholar]
- Marquet, A.; Gaudry, M. 1-bromo-3-methyl-2-butanone. Org. Synth. 1976, 55, 24. [Google Scholar] [CrossRef]
- Sorrell, T.N.; Allen, W.E. A Regiospecific Synthesis of 1,4-Disubstituted Imidazoles. J. Org. Chem. 1994, 59, 1589–1590. [Google Scholar] [CrossRef]
- Sorrell, T.N.; Allen, W.E.; Parkin, G.; Kimblin, C. Tris[2-(1,4-Diisopropylimidazolyl]Phosphine. In Inorganic Syntheses; Darensbourg, M.Y., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 66–70. ISBN 978-0-470-13263-0. [Google Scholar]
- Howson, S.E.; Allan, L.E.N.; Chmel, N.P.; Clarkson, G.J.; Deeth, R.J.; Faulkner, A.D.; Simpson, D.H.; Scott, P. Origins of Stereoselectivity in Optically Pure Phenylethaniminopyridinetris-Chelates M(NN′)3n+ (M = Mn, Fe, Co, Ni and Zn). Dalton Trans. 2011, 40, 10416–10433. [Google Scholar] [CrossRef] [PubMed]
- Ferri, N.; Cazzaniga, S.; Mazzarella, L.; Curigliano, G.; Lucchini, G.; Zerla, D.; Gandolfi, R.; Facchetti, G.; Pellizzoni, M.; Rimoldi, I. Cytotoxic Effect of (1-Methyl-1H-Imidazol-2-Yl)-Methanamine and Its Derivatives in PtII Complexes on Human Carcinoma Cell Lines: A Comparative Study with Cisplatin. Bioorg. Med. Chem. 2013, 21, 2379–2386. [Google Scholar] [CrossRef] [PubMed]
- Seto, M.; Miyamoto, N.; Aikawa, K.; Aramaki, Y.; Kanzaki, N.; Iizawa, Y.; Baba, M.; Shiraishi, M. Orally Active CCR5 Antagonists as Anti-HIV-1 Agents. Part 3: Synthesis and Biological Activities of 1-Benzazepine Derivatives Containing a Sulfoxide Moiety. Bioorg. Med. Chem. 2005, 13, 363–386. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaynor, R.B.; McIntyre, B.N.; Creutz, S.E. (4-(Adamantan-1-yl)-1-(isopropyl)-1H-imidazol-2-yl)methanol. Molbank 2023, 2023, M1566. https://doi.org/10.3390/M1566
Gaynor RB, McIntyre BN, Creutz SE. (4-(Adamantan-1-yl)-1-(isopropyl)-1H-imidazol-2-yl)methanol. Molbank. 2023; 2023(1):M1566. https://doi.org/10.3390/M1566
Chicago/Turabian StyleGaynor, Ryan B., Baylee N. McIntyre, and Sidney E. Creutz. 2023. "(4-(Adamantan-1-yl)-1-(isopropyl)-1H-imidazol-2-yl)methanol" Molbank 2023, no. 1: M1566. https://doi.org/10.3390/M1566