(Z)-3-(Dicyanomethylene)-4-((5-fluoro-3,3-dimethyl-1-(3-phenylpropyl)-3H-indol-1-ium-2-yl) methylene)-2-(((E)-5-fluoro-3,3-dimethyl-1-(3-phenylpropyl)indolin-2-ylidene)methyl) cyclobut-1-en-1-olate
Abstract
:1. Introduction
2. Results and Discussion
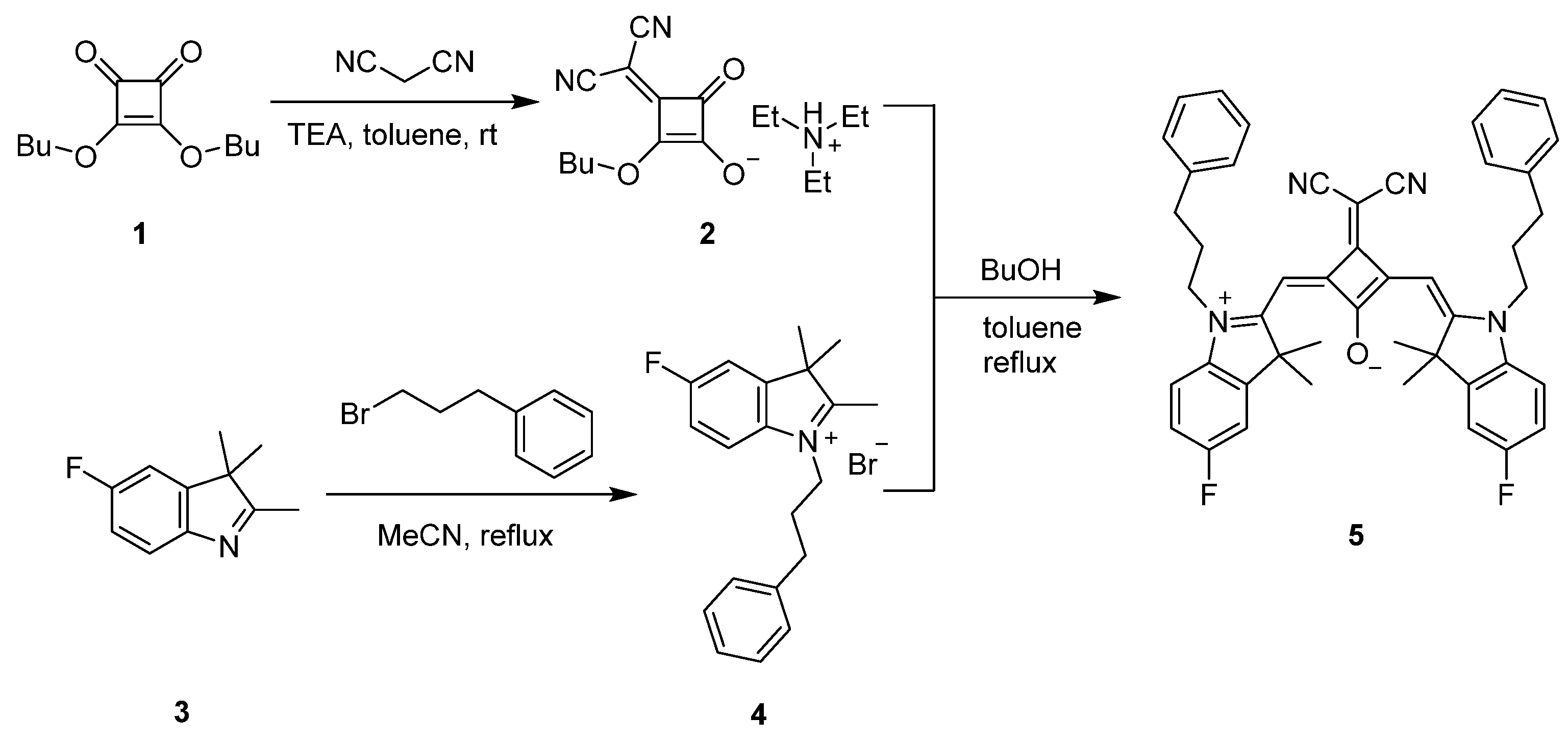
2.1. Synthesis
2.2. Physicochemical Properties of Dye 5 Compared to Commercially Available Squaraine (SQ)
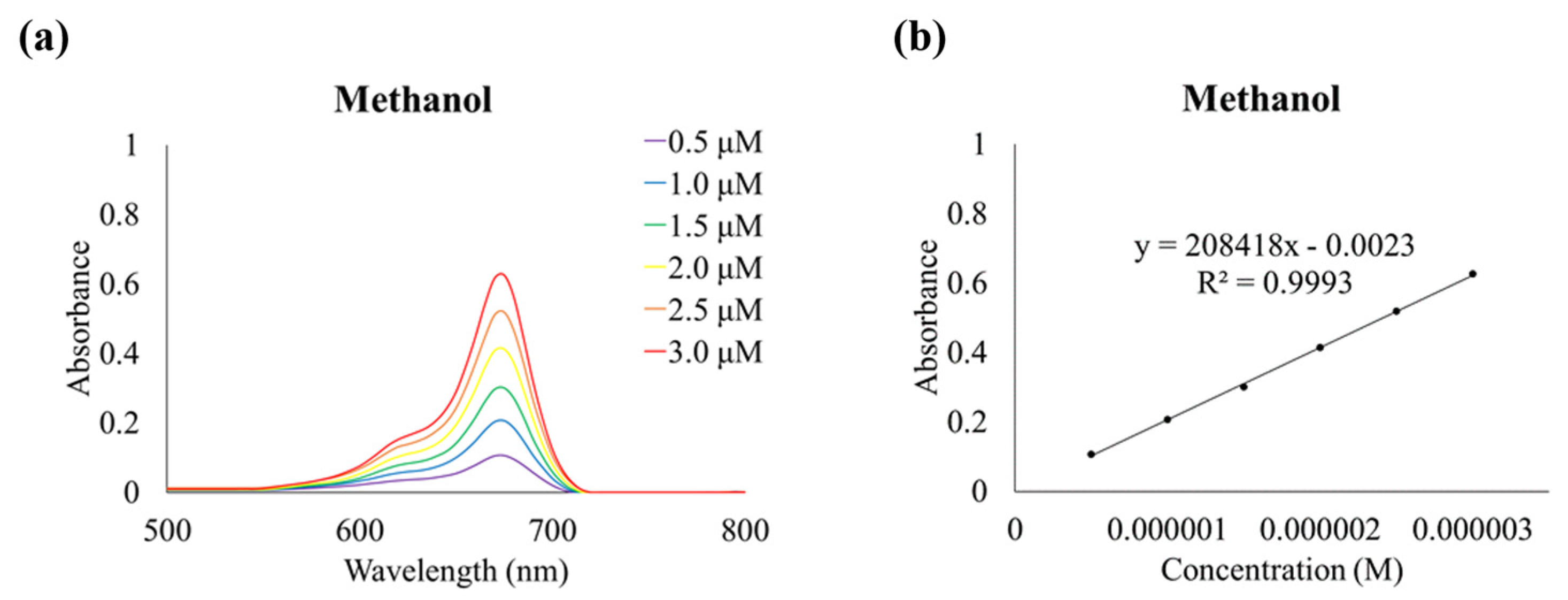
2.3. Optical Properties of Dye 5
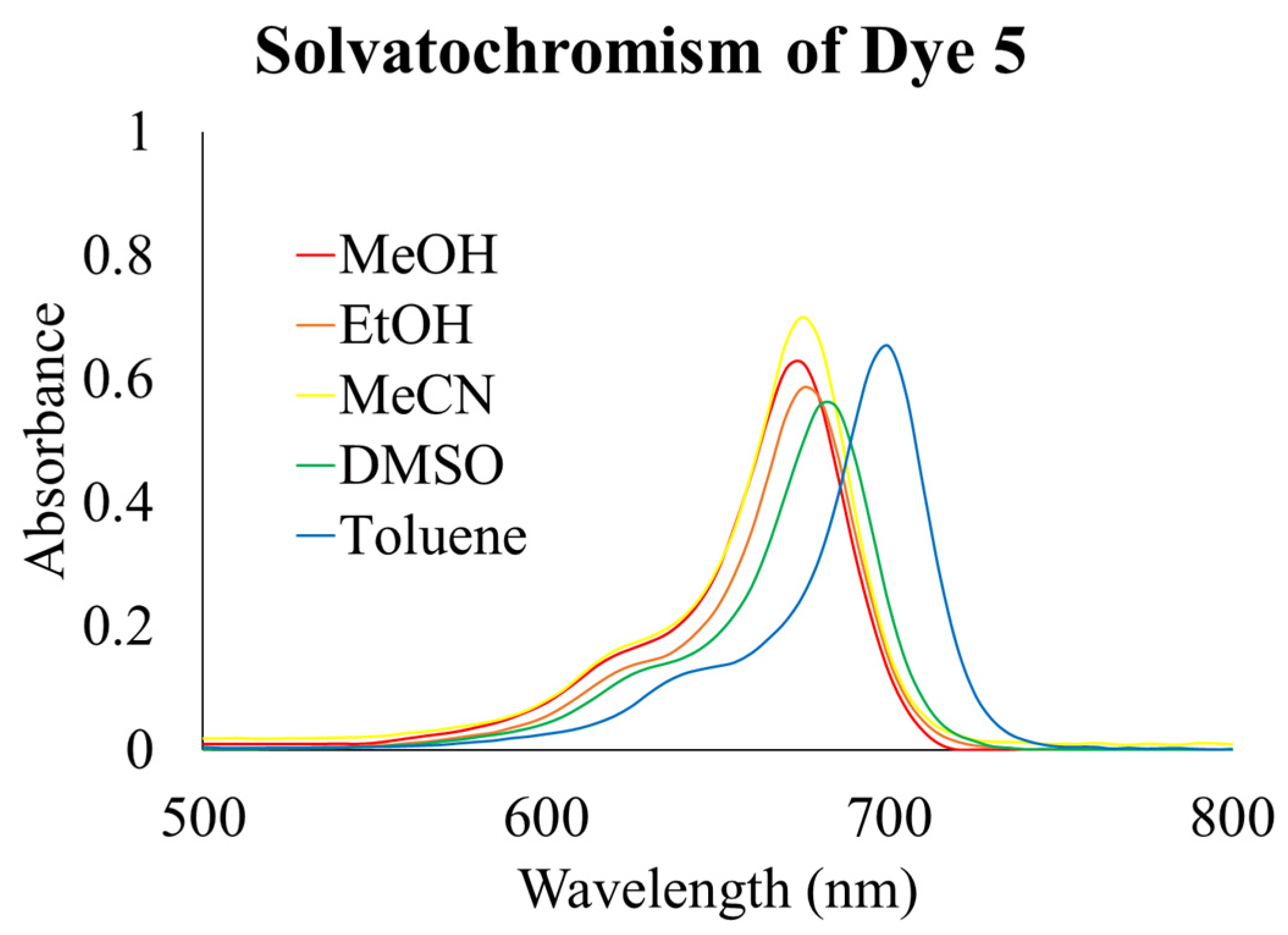
Solvatochromism of Dye 5
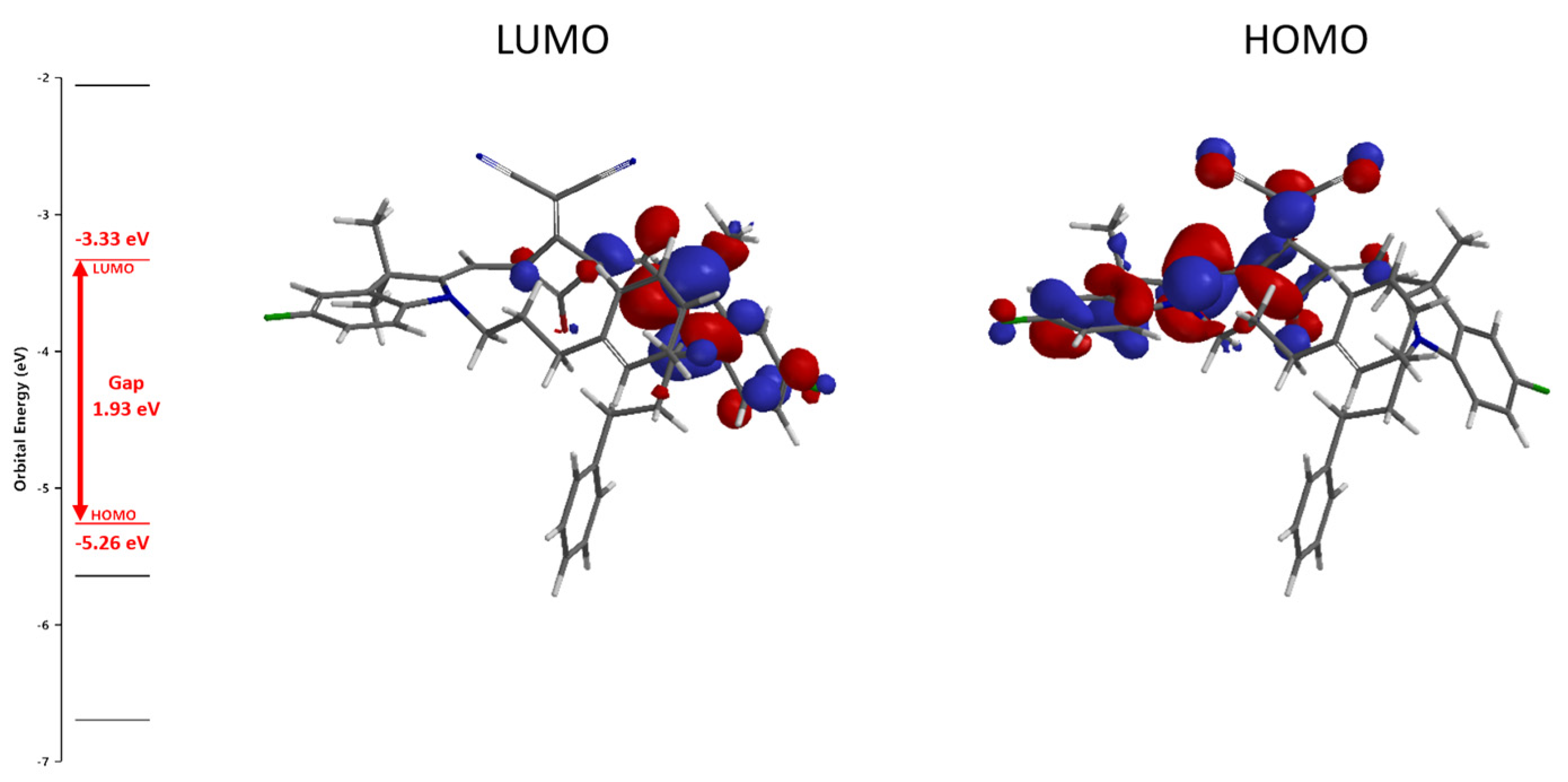
2.4. Density Functional Theory Calculations of Dye 5
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.3. Analytical Instrumentation
3.4. Analytical Instrumentation
3.5. Determination of Molar Absorptivity
3.6. Optical Hydrophobicity Studies
3.7. Physicochemical Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ilina, K.; MacCuaig, W.M.; Laramie, M.; Jeouty, J.N.; McNally, L.R.; Henary, M. Squaraine Dyes: Molecular Design for Different Applications and Remaining Challenges. Bioconjugate Chem. 2020, 31, 194–213. [Google Scholar] [CrossRef]
- Yao, D.; Wang, Y.; Zou, R.; Bian, K.; Liu, P.; Shen, S.; Yang, W.; Zhang, B.; Wang, D. Molecular Engineered Squaraine Nanoprobe for NIR-II/Photoacoustic Imaging and Photothermal Therapy of Metastatic Breast Cancer. ACS Appl. Mater. Interfaces 2020, 12, 4276–4284. [Google Scholar] [CrossRef]
- Sreejith, S.; Joseph, J.; Lin, M.; Menon, N.V.; Borah, P.; Ng, H.J.; Loong, Y.X.; Kang, Y.; Yu, S.W.-K.; Zhao, Y. Near-Infrared Squaraine Dye Encapsulated Micelles for in Vivo Fluorescence and Photoacoustic Bimodal Imaging. ACS Nano 2015, 9, 5695–5704. [Google Scholar] [CrossRef]
- Serpe, L.; Ellena, S.; Barbero, N.; Foglietta, F.; Prandini, F.; Gallo, M.P.; Levi, R.; Barolo, C.; Canaparo, R.; Visentin, S. Squaraines bearing halogenated moieties as anticancer photosensitizers: Synthesis, characterization and biological evaluation. Eur. J. Med. Chem. 2016, 113, 187–197. [Google Scholar] [CrossRef]
- Alessi, A.; Salvalaggio, M.; Ruzzon, G. Rhodamine 800 as reference substance for fluorescence quantum yield measurements in deep red emission range. J. Lumin. 2013, 134, 385–389. [Google Scholar] [CrossRef]
- Yadav, Y.; Owens, E.; Nomura, S.; Fukuda, T.; Baek, Y.; Kashiwagi, S.; Choi, H.S.; Henary, M. Ultrabright and Serum-Stable Squaraine Dyes. J. Med. Chem. 2020, 63, 9436–9445. [Google Scholar] [CrossRef]
- Galliano, S.; Novelli, V.; Barbero, N.; Smarra, A.; Viscardi, G.; Borrelli, R.; Sauvage, F.; Barolo, C. Dicyanovinyl and Cyano-Ester Benzoindolenine Squaraine Dyes: The Effect of the Central Functionalization on Dye-Sensitized Solar Cell Performance. Energies 2016, 9, 486. [Google Scholar] [CrossRef]
- Wu, J.; Yang, D.; Wang, Q.; Yang, L.; Sasabe, H.; Sano, T.; Kido, J.; Lu, Z.; Huang, Y. Central dicyanomethylene-substituted unsymmetrical squaraines and their application in organic solar cells. J. Mater. Chem. A 2018, 6, 5797–5806. [Google Scholar] [CrossRef]
- Wei, Y.; Hu, X.; Shen, L.; Jin, B.; Liu, X.; Tan, W.; Shangguan, D. Dicyanomethylene Substituted Benzothiazole Squaraines: The Efficiency of Photodynamic Therapy In Vitro and In Vivo. EBioMedicine 2017, 23, 25–33. [Google Scholar] [CrossRef]
- dos Santos Pisoni, D.; Petzhold, C.L.; de Abreu, M.P.; Rodembusch, F.S.; Campo, L.F. Synthesis, spectroscopic characterization and photophysical study of dicyanomethylene-substituted squaraine dyes. Comptes Rendus Chim. 2012, 15, 454–462. [Google Scholar] [CrossRef]
- Qin, C.; Numata, Y.; Zhang, S.; Yang, X.; Islam, A.; Zhang, K.; Chen, H.; Han, L. Novel Near-Infrared Squaraine Sensitizers for Stable and Efficient Dye-Sensitized Solar Cells. Adv. Funct. Mater. 2014, 24, 3059–3066. [Google Scholar] [CrossRef]
- Mayerhöffer, U.; Fimmel, B.; Würthner, F. Bright Near-Infrared Fluorophores Based on Squaraines by Unexpected Halogen Effects. Angew. Chem. Int. Ed. 2012, 51, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Casa, S.; Henary, M. Synthesis and Applications of Selected Fluorine-Containing Fluorophores. Molecules 2021, 26, 1160. [Google Scholar] [CrossRef] [PubMed]
- Barcenas, G.; Biaggne, A.; Mass, O.A.; Wilson, C.K.; Obukhova, O.M.; Kolosova, O.S.; Tatarets, A.L.; Terpetschnig, E.; Pensack, R.D.; Lee, J.; et al. First-principles studies of substituent effects on squaraine dyes. RSC Adv. 2021, 11, 19029–19040. [Google Scholar] [CrossRef]
- Owens, E.A.; Hyun, H.; Dost, T.L.; Lee, J.H.; Park, G.; Pham, D.H.; Park, M.H.; Choi, H.S.; Henary, M. Near-Infrared Illumination of Native Tissues for Image-Guided Surgery. J. Med. Chem. 2016, 59, 5311–5323. [Google Scholar] [CrossRef]
- Owens, E.A.; Tawney, J.G.; Henary, M.M. 2-{(E)-2-[(3E)-2-Chloro-3-{(2E)-2-[1,1-dimethyl-3-(3-phenylpropyl)-1,3-dihydro-2H-benzo[e]indol-2-ylidene]-ethylidene}cyclohex-1-en-1-yl]ethenyl}-1,1-dimethyl-3-(3-phenylpropyl)-1H-benzo[e]indolium Iodide. Molbank 2014, 2014, M814. [Google Scholar] [CrossRef]
- Aristova, D.; Volynets, G.; Chernii, S.; Losytskyy, M.; Balanda, A.; Slominskii, Y.; Mokhir, A.; Yarmoluk, S.; Kovalska, V. Far-red pentamethine cyanine dyes as fluorescent probes for the detection of serum albumins. R Soc. Open Sci. 2020, 7, 200453. [Google Scholar] [CrossRef]
- Levitz, A.; Marmarchi, F.; Henary, M. Synthesis and Optical Properties of Near-Infrared meso-Phenyl-Substituted Symmetric Heptamethine Cyanine Dyes. Molecules 2018, 23, 226. [Google Scholar] [CrossRef]
- Biffinger, J.C.; Kim, H.W.; DiMagno, S.G. The Polar Hydrophobicity of Fluorinated Compounds. ChemBioChem 2004, 5, 622–627. [Google Scholar] [CrossRef]
- Buabeng, E.R.; Henary, M. 2-((E)-2-((E)-4-Chloro-5-(2-((E)-5-methoxy-3,3-dimethyl-1-(3-phenylpropyl)indolin-2-ylidene) ethylidene)-1,1-dimethyl-1,2,5,6-tetrahydropyridin-1-ium-3-yl)vinyl)-5-methoxy-3,3-dimethyl-1-(3-phenylpropyl)-3H-indol-1-ium. Molbank 2021, 2021, M1270. [Google Scholar] [CrossRef]
- Essam, Z.M.; Ozmen, G.E.; Setiawan, D.; Hamid, R.R.; Abd El-Aal, R.M.; Aneja, R.; Hamelberg, D.; Henary, M. Donor acceptor fluorophores: Synthesis, optical properties, TD-DFT and cytotoxicity studies. Org. Biomol. Chem. 2021, 19, 1835–1846. [Google Scholar] [CrossRef] [PubMed]
- Nigam, S.; Rutan, S. Principles and Applications of Solvatochromism. Appl. Spectrosc. 2001, 55, 362A–370A. [Google Scholar] [CrossRef]



| Dye | LogD (pH 7.4) | Polarizability | Dipole Moment (Debye) | Rotatable Bonds | MV (Å3) | MW (g/mol) | MSA (Å3) |
|---|---|---|---|---|---|---|---|
| SQ | 2.96 | 49.01 | 9.77 | 2 | 394.21 | 424.54 | 613.90 |
| 5 | 7.31 | 79.59 | 15.34 | 10 | 654.11 | 716.88 | 1001.06 |
| Solvent | λabs (nm) | λem (nm) | Stokes Shift (nm) | ε (L mol −1 cm−1) | ΦF (%) |
|---|---|---|---|---|---|
| Methanol | 675 | 691 | 16 | 208,000 | 25 |
| Ethanol | 675 | 693 | 18 | 188,000 | 52 |
| Acetonitrile | 675 | 691 | 16 | 235,000 | 12 |
| Toluene | 700 | 714 | 14 | 222,000 | 63 |
| DMSO | 680 | 698 | 18 | 188,000 | 34 |
| Rhodamine800 [5] | 682 | 700 | 18 | 113,000 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casa, S.; Ersoy Ozmen, G.; Henary, M. (Z)-3-(Dicyanomethylene)-4-((5-fluoro-3,3-dimethyl-1-(3-phenylpropyl)-3H-indol-1-ium-2-yl) methylene)-2-(((E)-5-fluoro-3,3-dimethyl-1-(3-phenylpropyl)indolin-2-ylidene)methyl) cyclobut-1-en-1-olate. Molbank 2023, 2023, M1576. https://doi.org/10.3390/M1576
Casa S, Ersoy Ozmen G, Henary M. (Z)-3-(Dicyanomethylene)-4-((5-fluoro-3,3-dimethyl-1-(3-phenylpropyl)-3H-indol-1-ium-2-yl) methylene)-2-(((E)-5-fluoro-3,3-dimethyl-1-(3-phenylpropyl)indolin-2-ylidene)methyl) cyclobut-1-en-1-olate. Molbank. 2023; 2023(1):M1576. https://doi.org/10.3390/M1576
Chicago/Turabian StyleCasa, Stefanie, Guliz Ersoy Ozmen, and Maged Henary. 2023. "(Z)-3-(Dicyanomethylene)-4-((5-fluoro-3,3-dimethyl-1-(3-phenylpropyl)-3H-indol-1-ium-2-yl) methylene)-2-(((E)-5-fluoro-3,3-dimethyl-1-(3-phenylpropyl)indolin-2-ylidene)methyl) cyclobut-1-en-1-olate" Molbank 2023, no. 1: M1576. https://doi.org/10.3390/M1576
APA StyleCasa, S., Ersoy Ozmen, G., & Henary, M. (2023). (Z)-3-(Dicyanomethylene)-4-((5-fluoro-3,3-dimethyl-1-(3-phenylpropyl)-3H-indol-1-ium-2-yl) methylene)-2-(((E)-5-fluoro-3,3-dimethyl-1-(3-phenylpropyl)indolin-2-ylidene)methyl) cyclobut-1-en-1-olate. Molbank, 2023(1), M1576. https://doi.org/10.3390/M1576








