2-[2,6-Diisopropylphenyl]-4-phenyl-5H-5,9b[1′,2′]-benzonaphtho[1,2-b]pyrrol-2-ium Tetrafluoroborate
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Considerations
3.2. Synthesis of N-(Anthracen-9-ylmethyl)-2,6-diisopropylaniline (2)
3.3. Synthesis of 2-[2,6-Diisopropylphenyl]-4-phenyl-5h-5,9b[1′,2′]-benzonaphtho[1,2-b]pyrrol-2-ium Tetrafluoroborate (3)
3.4. Synthesis of (Z)-Anthracenyl-9-methyl-(2,6-diisopropylphenyl)-(3-phenylprop-2-ynylidene)ammonium Tetrafluoroborate [(Z)-4]
3.5. SCXRD Analysis of 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Layer, R.W. The Chemistry of Imines. Chem. Rev. 1963, 63, 489–510. [Google Scholar] [CrossRef]
- Erkkilä, A.; Majander, I.; Pihko, P.M. Iminium Catalysis. Chem. Rev. 2007, 107, 5416–5470. [Google Scholar] [CrossRef] [PubMed]
- Emmelius, M.; Pawlowski, G.; Vollmann, H.W. Materials for Optical Data Storage. Angew. Chem. Int. Ed. Engl. 1989, 28, 1445–1471. [Google Scholar] [CrossRef]
- Mustroph, H.; Stollenwerk, M.; Bressau, V. Current Developments in Optical Data Storage with Organic Dyes. Angew. Chem. Int. Ed. 2006, 45, 2016–2035. [Google Scholar] [CrossRef]
- Sun, W.; Guo, S.; Hu, C.; Fan, J.; Peng, X. Recent Development of Chemosensors Based on Cyanine Platforms. Chem. Rev. 2016, 116, 7768–7817. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Shi, L.; Zhang, B.; Liu, L.; Fu, Y.; Zhang, X. Recent advances in bioprobes and biolabels based on cyanine dyes. Anal. Bioanal. Chem. 2022, 414, 4551–4573. [Google Scholar] [CrossRef] [PubMed]
- Pronkin, P.G.; Tatikolov, A.S. Photonics of Trimethine Cyanine Dyes as Probes for Biomolecules. Molecules 2022, 27, 6367. [Google Scholar] [CrossRef] [PubMed]
- Tigreros, A.; Rosero, H.-A.; Castillo, J.-C.; Portilla, J. Integrated pyrazolo[1,5-a]pyrimidine-hemicyanne system as a colorimetric and fluorometric chemosensor for cyanide recognition in water. Talanta 2019, 196, 395–401. [Google Scholar] [CrossRef]
- Ishii, A.; Nakata, N.J. Synthesis and Photophysical Property of 1-Chalcogeno-1,3-butadiene Derivatives and the Related Compounds Incorporated in a Dibenzobarrelene Skeleton. Synth. Org. Chem. Jpn. 2018, 76, 1042–1054. [Google Scholar] [CrossRef]
- Ishii, A.; Shibata, M.; Ebina, R.; Nakata, N. Synthesis and Photophysical Properties of Dibenzobarrelene-Incorporated 1,4-Diphenyl-1,3-pentadienes and a 5-Sila Derivative Having High Fluorescence Efficiency. Eur. J. Org. Chem. 2018, 1011–1018. [Google Scholar] [CrossRef]
- Ishii, A.; Kikushima, C.; Hayashi, Y.; Ohtsuka, N.; Nakata, N.; Muranaka, A.; Tanaka, Y.; Uchiyama, M. 1-Phosphino-1,3-butadiene Derivatives Incorporated with Dibenzobarrelene Skeleton: Synthesis and Photophysical Properties. Bull. Chem. Soc. Jpn. 2020, 93, 1430–1442. [Google Scholar] [CrossRef]
- Miyashita, Y.; Nakata, N.; Ishii, A. Synthesis and Properties of 1-(Dialkylstannyl)-1,4-diphenyl-1,3-butadiene Fused with a Dibenzobarrelene and the Corresponding Pentaorganostannate. Z. Anorg. Allg. Chem. 2021, 647, 1883–1889. [Google Scholar] [CrossRef]
- Ishii, A.; Ebina, R.; Nakata, N. Formation, Chemical and Optical Properties of 1,2,5-Triphenylpentadienyl Cation Fixed in a Rigid Dibenzobarrelene Skeleton. Eur. J. Org. Chem. 2022, 2022, e202200033. [Google Scholar] [CrossRef]
- Zhou, J.; Li, X.Y.; Sun, H.J. An efficient and recyclable water-soluble cyclopalladated complex for aqueous Suzuki reactions under aerial conditions. J. Organomet. Chem. 2010, 695, 297–303. [Google Scholar] [CrossRef]
- Kuroda, H.; Hanaki, E.; Izawa, H.; Kano, M.; Itahashi, H. A convenient method for the preparation of α-vinylfurans by phosphine-initiated reactions of various substituted enynes bearing a carbonyl group with aldehydes. Tetrahedron 2004, 60, 1913–1920. [Google Scholar] [CrossRef]
- Lavallo, V.; Canac, Y.; Präsang, C.; Donnadieu, B.; Bertrand, G. Stable Cyclic (Alkyl)(Amino)Carbenes as Rigid or Flexible, Bulky, Electron-Rich Ligands for Transition-Metal Catalysts: A Quaternary Carbon Atom Makes the Difference. Angew. Chem., Int. Ed. 2005, 44, 5705–5709. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Jazzar, R.; Dewhurst, R.D.; Bourg, J.-B.; Donnadieu, B.; Canac, Y.; Bertrand, G. Intramolecular ″Hydroiminiumation″ of Alkenes: Application to the Synthesis of Conjugate Acids of Cyclic Alkyl Amino Carbenes (CAACs). Angew. Chem. Int. Ed. 2007, 46, 2899–2902. [Google Scholar] [CrossRef]
- Jazzar, R.; Dewhurst, R.D.; Bourg, J.-B.; Donnadieu, B.; Canac, Y.; Bertrand, G. Intramolecular ″Hydroiminiumation and -Amidiniumation″ of Alkenes: A Convenient, Flexible, and Scalable Route to Cyclic Iminium and Imidazolinium Salts. J. Org. Chem. 2007, 72, 3492–3499. [Google Scholar] [CrossRef]
- Dureen, M.A.; Brown, C.C.; Stephan, D.W. Addition of enamines or pyrroles and B(C6F5)3 “Frustrated Lewis Pairs” to alkynes. Organometallics 2010, 29, 6422–6432. [Google Scholar] [CrossRef]
- Smolobochkin, A.V.; Gazizov, A.S.; Melyashova, A.S.; Voronina, J.K.; Strelnik, A.G.; Vatsadze, S.Z.; Burilov, A.R.; Pudovik, M.A.; Fedorova, O.A.; Sinyashin, O.G. Tandem intramolecular cyclisation/1,3-aryl shift in N-(4,4-diethoxybutyl)-1-arylmethanimines (Kazan reaction): Synthesis of 3-benzylidene-1-pyrrolines. RSC Adv. 2017, 7, 50955–50960. [Google Scholar] [CrossRef]
- Lombardi, B.M.P.; Pezoulas, E.R.; Suvinen, R.A.; Harrison, A.; Dubrawski, Z.S.; Gelfand, B.S.; Tuononen, H.M.; Roesler, R. Bis[cyclic (alkyl)(amino)carbene] isomers: Stable trans-bis(CAAC) versus facile olefin formation for cis-bis(CAAC). Chem. Commun. 2022, 58, 6482–6485. [Google Scholar] [CrossRef] [PubMed]
- Vermersch, F.; Oliveira, L.; Hunter, J.; Soleilhavoup, M.; Jazzar, R.; Bertrand, G. Cyclic (Alkyl)(Amino)Carbenes: Synthesis of Iminium Precursors and Structural Properties. J. Org. Chem. 2022, 87, 3511–3518. [Google Scholar] [CrossRef] [PubMed]
- Olguín, J.; Bernès, S.; Gasque, L. Fluoride Ion as Ligand and Hydrogen Bond Acceptor: Crystal Structures of Two Dinuclear Cuii Complexes Built on a Diazecine Template. Crystals 2012, 2, 1357–1365. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef]


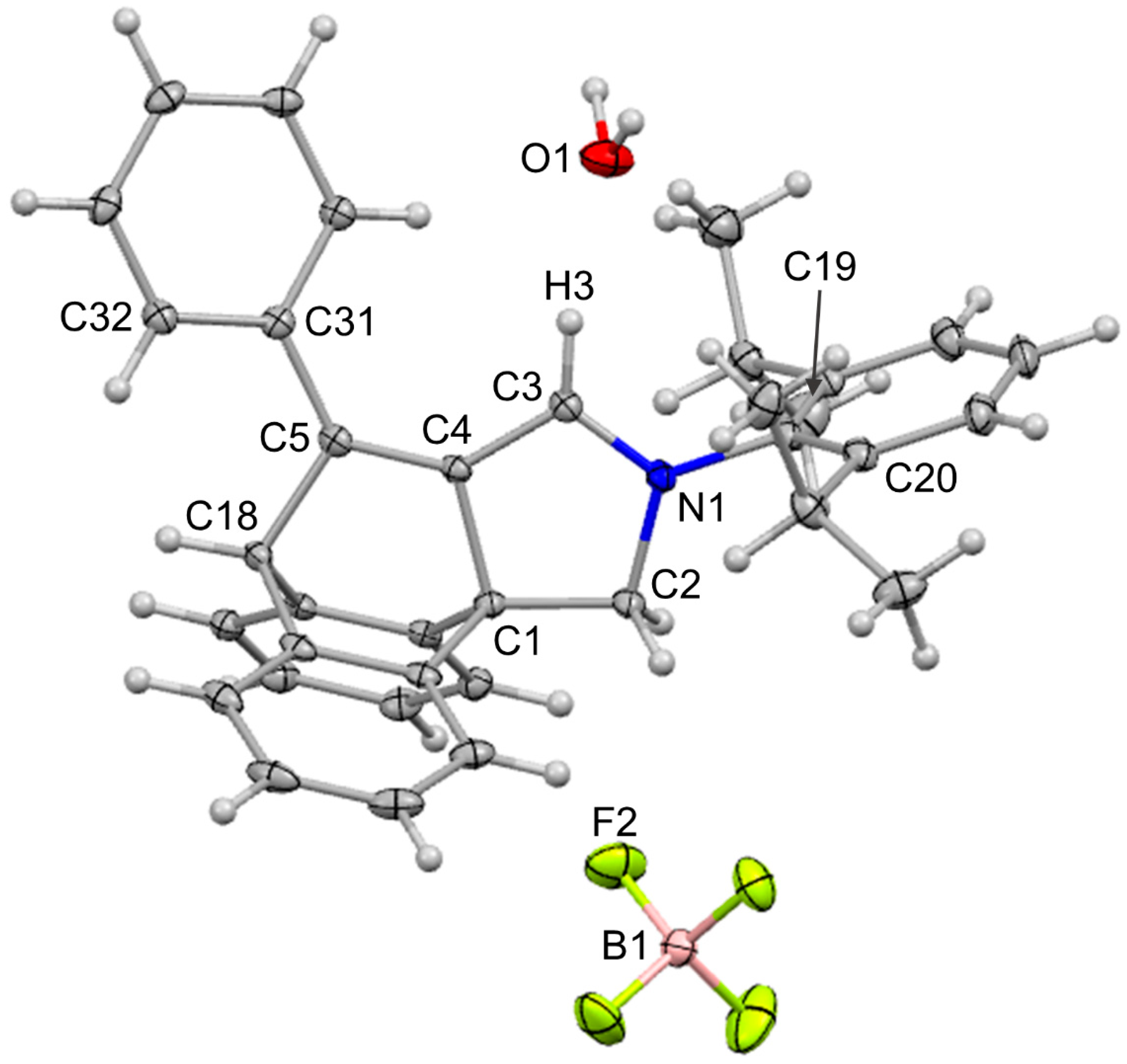

| Bond Lengths | [Å] | Bond Angles | [°] |
|---|---|---|---|
| C4–C5 | 1.355(2) | N1–C3–C4 | 111.67(16) |
| C3–C4 | 1.409(2) | C3–C4–C1 | 107.34(15) |
| C3–N1 | 1.308(2) | C3–C4–C5 | 135.48(16) |
| C2–N1 | 1.484(2) | C1–C4–C5 | 117.10(15) |
| C1–C2 | 1.521(2) | C4–C5–C18 | 110.06(15) |
| C1–C4 | 1.547(3) | C31–C5–C4 | 128.92(16) |
| H3…O1 | 2.1845(17) | C3–N1–C19–C20 | −77.0(2) |
| B1…N1 | 5.194(2) | C4–C5–C31–C32 | −153.38(17) |
| N1–C3–C4–C5 | 179.34(18) |
| λabs [nm] | ε [M−1cm−1] | λem (CH2Cl2) [nm] | Stokes Shift [cm−1] (nm) | ΦF (CH2Cl2) b | λem (solid) [nm] | ΦF (Solid) b |
|---|---|---|---|---|---|---|
| 423 | 9820 | 516 | 4300 (152) | 0.63 | 517 | 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, M.; Kamiyama, S.; Ishii, A.; Nakata, N. 2-[2,6-Diisopropylphenyl]-4-phenyl-5H-5,9b[1′,2′]-benzonaphtho[1,2-b]pyrrol-2-ium Tetrafluoroborate. Molbank 2023, 2023, M1601. https://doi.org/10.3390/M1601
Tanaka M, Kamiyama S, Ishii A, Nakata N. 2-[2,6-Diisopropylphenyl]-4-phenyl-5H-5,9b[1′,2′]-benzonaphtho[1,2-b]pyrrol-2-ium Tetrafluoroborate. Molbank. 2023; 2023(1):M1601. https://doi.org/10.3390/M1601
Chicago/Turabian StyleTanaka, Masaru, Shota Kamiyama, Akihiko Ishii, and Norio Nakata. 2023. "2-[2,6-Diisopropylphenyl]-4-phenyl-5H-5,9b[1′,2′]-benzonaphtho[1,2-b]pyrrol-2-ium Tetrafluoroborate" Molbank 2023, no. 1: M1601. https://doi.org/10.3390/M1601
APA StyleTanaka, M., Kamiyama, S., Ishii, A., & Nakata, N. (2023). 2-[2,6-Diisopropylphenyl]-4-phenyl-5H-5,9b[1′,2′]-benzonaphtho[1,2-b]pyrrol-2-ium Tetrafluoroborate. Molbank, 2023(1), M1601. https://doi.org/10.3390/M1601







