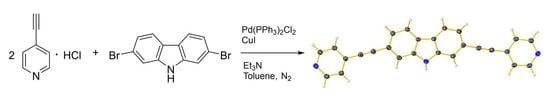
2,7-Bis(pyridin-4-ylethynyl)-9H-carbazole
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Synthesis of 2,7-Bis(pyridin-4-ylethynyl)-9H-carbazole (1)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Podda, E.; Arca, M.; Pintus, A.; Lippolis, V.; Caltagirone, C.; Coles, S.; Orton, J.B.; Ennas, G.; Picci, G.; Davies, R.; et al. On the Role of Torsional Dynamics in the Solid-State Fluorescent Properties of a New Bifluorene-Tetracarboxylic Acid and Its Supramolecular Assemblies: A Structural and TD-DFT Investigation. CrystEngComm 2023, 25, 1058–1066. [Google Scholar] [CrossRef]
- Ma, T.; Li, K.; Hu, J.; Xin, Y.; Cao, J.; He, J.; Xu, Z. Carbazole-Equipped Metal-Organic Framework for Stability, Photocatalysis, and Fluorescence Detection. Inorg. Chem. 2022, 61, 14352–14360. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.C.; Evenson, S.J.; McCausland, C.B. Fluorescent Thiophene-Based Materials and Their Outlook for Emissive Applications. Chem. Commun. 2015, 51, 4528–4543. [Google Scholar] [CrossRef] [PubMed]
- Konidena, R.K.; Thomas, K.R.J.; Park, J.W. Recent Advances in the Design of Multi-Substituted Carbazoles for Optoelectronics: Synthesis and Structure-Property Outlook. ChemPhotoChem 2022, 6, e202200059. [Google Scholar] [CrossRef]
- Qiao, J.; Liu, X.; Zhang, L.; Eubank, J.F.; Liu, X.; Liu, Y. Unique Fluorescence Turn-On and Turn-Off-On Responses to Acids by a Carbazole-Based Metal-Organic Framework and Theoretical Studies. J. Am. Chem. Soc. 2022, 144, 17054–17063. [Google Scholar] [CrossRef]
- Palamà, I.; Di Maria, F.; Viola, I.; Fabiano, E.; Gigli, G.; Bettini, C.; Barbarella, G. Live-Cell-Permeant Thiophene Fluorophores and Cell-Mediated Formation of Fluorescent Fibrils. J. Am. Chem. Soc. 2011, 133, 17777–17785. [Google Scholar] [CrossRef]
- Chuang, P.M.; Wu, J.Y. A Highly Stable Zn Coordination Polymer Exhibiting pH-Dependent Fluorescence and as a Visually Ratiometric and on–off Fluorescent Sensor. CrystEngComm 2021, 23, 5226–5240. [Google Scholar] [CrossRef]
- Chuang, P.M.; Huang, Y.W.; Liu, Y.L.; Wu, J.Y. The Influence of Linker Substitution on the Fluorescence Responsive Sensing of Isostructural Coordination Polymers: Visual Turn-on, Ratiometric, and Turn-off Sensing in Water. CrystEngComm 2021, 23, 2222–2234. [Google Scholar] [CrossRef]
- Song, Y.H.; Singh, N.; Jung, J.; Kim, H.; Kim, E.H.; Cheong, H.K.; Kim, Y.; Chi, K.W. Template-Free Synthesis of a Molecular Solomon Link by Two-Component Self-Assembly. Angew. Chem. Int. Ed. 2016, 55, 2007–2011. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wang, Z.K.; Lang, F.F.; Yu, H.M.; Cao, C.; Ni, C.Y.; Wang, M.Y.; Song, Y.L.; Lang, J.P. Construction of Cluster-Based Supramolecular Wire and Rectangle. Dalton Trans. 2022, 51, 6358–6365. [Google Scholar] [CrossRef] [PubMed]
- Podda, E.; Coles, S.J.; Horton, P.N.; Lickiss, P.D.; Bull, O.S.; Orton, J.B.; Pintus, A.; Pugh, D.; Aragoni, M.C.; Davies, R.P. First Example of Solid-State Luminescent Borasiloxane-Based Chiral Helices Assembled through N-B Bonds. Dalton Trans. 2021, 50, 3782–3785. [Google Scholar] [CrossRef]
- Podda, E.; Arca, M.; Pintus, A.; Demontis, V.; Lippolis, V.; Ferino, G.; Orton, J.B.; Coles, S.J.; Aragoni, M.C. 2,7-Bis(Pyridin-3-Ylethynyl)Fluoren-9-One. Molbank 2023, 2023, M1540. [Google Scholar] [CrossRef]
- Podda, E.; Arca, M.; Pintus, A.; Meloni, F.; Lippolis, V.; Ferino, G.; Orton, J.B.; Coles, S.J.; Aragoni, M.C. 2-(2,7-Bis(Pyridin-3-Ylethynyl)Fluoren-9-Ylidene)Malononitrile. Molbank 2023, 2023, M1619. [Google Scholar] [CrossRef]
- Aragoni, M.C.; Arca, M.; Coles, S.J.; Crespo Alonso, M.; Coles, S.L.; Davies, R.P.; Hursthouse, M.B.; Isaia, F.; Lai, R.; Lippolis, V. Coordination Polymers and Polygons Using Di-Pyridyl-Thiadiazole Spacers and Substituted Phosphorodithioato NiII Complexes: Potential and Limitations for Inorganic Crystal Engineering. CrystEngComm 2016, 18, 5620–5629. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podda, E.; Arca, M.; Pintus, A.; Lippolis, V.; Ferino, G.; Orton, J.B.; Coles, S.J.; Aragoni, M.C. 2,7-Bis(pyridin-4-ylethynyl)-9H-carbazole. Molbank 2023, 2023, M1703. https://doi.org/10.3390/M1703
Podda E, Arca M, Pintus A, Lippolis V, Ferino G, Orton JB, Coles SJ, Aragoni MC. 2,7-Bis(pyridin-4-ylethynyl)-9H-carbazole. Molbank. 2023; 2023(3):M1703. https://doi.org/10.3390/M1703
Chicago/Turabian StylePodda, Enrico, Massimiliano Arca, Anna Pintus, Vito Lippolis, Giulio Ferino, James B. Orton, Simon J. Coles, and Maria Carla Aragoni. 2023. "2,7-Bis(pyridin-4-ylethynyl)-9H-carbazole" Molbank 2023, no. 3: M1703. https://doi.org/10.3390/M1703
APA StylePodda, E., Arca, M., Pintus, A., Lippolis, V., Ferino, G., Orton, J. B., Coles, S. J., & Aragoni, M. C. (2023). 2,7-Bis(pyridin-4-ylethynyl)-9H-carbazole. Molbank, 2023(3), M1703. https://doi.org/10.3390/M1703