Abstract
Isoamyl (E)-3-(3,4-dimethoxyphenyl)acrylate (1) was obtained from the 2-methyl-6-nitrobenzoic anhydride (MNBA)/4-dimethylaminopyridine (DMAP)-catalyzed reaction at room temperature for 190 min in dichloromethane with a yield of 95%. The structure of isoamyl (E)-3-(3,4-dimethoxyphenyl)acrylate (1) was elucidated using NMR, FTIR, and high-resolution mass spectrometry. In vitro sun protection factor evaluation exhibited a value of 37.10 ± 0.03 which indicates that isoamyl (E)-3-(3,4-dimethoxyphenyl)acrylate (1) is a sunscreen agent with high protection.
1. Introduction
Cinnamate derivatives are widely used in cosmetics as a fragrance [1], sunscreen [2], skin whitening agent [3], and anti-aging agent [4]. Esters of 4-methoxycinnamic acid, namely isoamyl 4-methoxycinnamate and 2-ethoxyethyl 4-methoxycinnamate, are compounds used in commercial sunscreens [5]. The photoprotection ability of sunscreen compounds can be enhanced by adding a methoxy group to increase the coverage of protection [6]. The photoprotective efficiency of a sunscreen can be indicated by the value of sun protection factor [7]. Esters as cinnamate derivatives are generally synthesized via esterification involving thionyl chloride as a coupling agent. However, thionyl chloride can be very harmful, indicated by its inclusion in the Chemical Weapons Convention list [8]. Synthesis of esters can also be carried out using N,N′-dicyclohexylcarbodiimide (DCC) and organocatalyst 4-dimethylaminopyridine (DMAP) as a coupling agent [9]. However, DCC is an irritant, allergen, and can harm internal organs [10]. Furthermore, N,N′-dicyclohexylurea, a resulting byproduct, is only moderately soluble in many organic solvents and insoluble in water, causing difficulties in purification [11]. On the other hand, the synthesis of carboxylic esters using 2-methyl-6-nitrobenzoic anhydride (MNBA) as a coupling agent can be achieved through a one-pot reaction at room temperature with high yields and good chemoselectivity [12]. We report the MNBA/DMAP-catalyzed synthesis of isoamyl (E)-3-(3,4-dimethoxyphenyl)acrylate (1) and investigated its photoprotective ability by determining its SPF value.
2. Results and Discussion
2.1. Chemistry
The synthesis of isoamyl (E)-3-(3,4-dimethoxyphenyl)acrylate (1) was carried out by utilizing the reaction of 3,4-dimethoxycinnamic acid and isoamyl alcohol at room temperature in the presence of MNBA and DMAP in anhydrous dichloromethane without inert condition (Scheme 1). There was a color change at the beginning of the reaction, from bright yellow to light yellow, then no color change until the end of the reaction. The crude product was purified by means of column chromatography to obtain isoamyl (E)-3-(3,4-dimethoxyphenyl)acrylate (1) in the form of a yellowish liquid with a yield of 95%. The reaction was carried out on a larger scale (1.55 g, 7.50 mmol) and gave almost the same yield of 94%. The 1H NMR spectrum of the synthesis product confirmed the absence of the carboxylic proton signal of the 3,4-dimethoxycinnamic acid and showed the presence of isoamyloxy group protons of the ester (1). The spectrum showed a doublet signal at a chemical shift (δ) 0.95 (J = 6.8 Hz) ppm, which was the signal of six protons from two methyl groups coupled to the protons of the methine group, which gave a multiplet signal at δ 1.72–1.74 ppm. A quartet signal can be observed at δ 1.60 (J = 6.8 Hz) ppm, which was the signal of two methylene group protons coupled to two neighboring methylene group protons, which gave a triplet signal at δ 4.23 (J = 6.8 Hz) ppm. The singlet signal at δ 3.91 ppm is the signal of six protons from the two methoxy groups, and the doublet signal at δ 6.30 (J = 16.0 Hz) ppm is the signal from an alkene proton coupled to a neighboring alkene proton, which gave a doublet signal at δ 7.62 (J = 16.0 Hz) ppm. The aromatic protons gave two doublet signals at δ 6.86 (J = 8.0 Hz) and 7.05 (J = 2.0 Hz) ppm, and a doublet doublet signal at 7.10 (J = 2.0; 8.0 Hz) ppm. The 13C spectrum showed fifteen signals corresponding to the number of carbon types present in compound (1). Signals at δ 22.59 and 25.18 ppm were those of the methyl and methine carbon groups, respectively. Signals at δ 37.55 and 63.19 ppm were signals of two methylene group carbons, and signals at δ 55.95 and 56.05 ppm were signals of two methoxy group carbons. The signals at δ 109.59, 111.06, 116.05, 122.68, 127.52, 144.57, 149.26, and 151.12 ppm were the signals of alkene and aromatic carbons. The signal at δ 167.43 ppm was the signal of the carbonyl ester carbon which was reinforced by the presence of strong absorption at wave number 1702 cm−1 in the infrared spectrum. Absorption at wave number 1157 cm−1 showed absorption of the C-O ester. The high-resolution mass spectrum further supported the synthesis product as isoamyl (E)-3-(3,4-dimethoxyphenyl)acrylate (1) which showed a peak ion [M + Na]+ with the molecular formula C16H22O4Na at m/z 301.1418 which was almost the same as the calculation result of 301.1416, a peak ion [M + H]+ with the molecular formula C16H23O4Na at m/z 279.1756 which was almost the same as the calculation result of 279.1596, a peak ion [2M + Na]+ with the molecular formula C32H44O4Na at m/z 579.1752 which was almost the same as the calculation result of 579.2934, and a cleavage of isoamyloxy radical from the molecular ion gave cation with a peak at m/z 191.1122 which was almost the same as the calculation result of 191.2027. Plausible mechanism for the formation of compound (1) can be seen in Supplementary Materials.
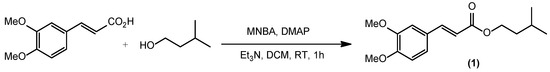
Scheme 1.
Synthesis of isoamyl (E)-3-(3,4-dimethoxyphenyl)acrylate (1).
2.2. In Vitro Sun Protection Factor Evaluation
The sun protection factor (SPF) value is a quantitative measure that indicates the effectiveness of a sunscreen formulation. SPF values are grouped into four categories based on their protection abilities, namely low (SPF 6–14.9), moderate (SPF 15–29.9), high (SPF 30–50), and very high (SPF 50–100) [13]. A sunscreen agent with an SPF value of 15 or more helps to protect skin from UVA and UV B sun ultraviolet radiation, and as a result reduces the risk of skin cancer and premature skin aging [14]. The SPF value was determined using the Mansur equation via spectrophotometry in the ultraviolet region at 290–320 nm [15]. Isoamyl (E)-3-(3,4-dimethoxyphenyl)acrylate (1) obtained an SPF value of 37.10 ± 0.03, which falls into the high category, indicating that it is a sunscreen agent with high protection. This was caused by the presence of an aromatic ring conjugated with C=C and C=O group, and two methoxy group as auxochrome attached to the aromatic ring [16]. This compound has three possible mechanisms when exposed to UV photon energy: (i) conformational molecular changes; (ii) emission of radiation at longer wavelengths; or (iii) release of incident energy as heat [17,18]. UV radiation is absorbed by the molecules which causes their photoexcitation into a form that has higher energy. Cinnamates adopt a stable trans (E) form in the electronic ground state (S0) which then isomerizes to the cis (Z) form. Furthermore, when this molecule returns to its initial state, energy is emitted in a form that is lower than the energy absorbed which provides sunscreen activity [19,20,21].
3. Materials and Methods
The chemicals and solvents were purchased from Sigma-Aldrich (St. Louis, MO, USA), Merck (Rahway, NJ, USA), and Fluka (Charlotte, NC, USA) and were used without further purification. Thin layer chromatography was carried out using Merck 0.20 mm precoated silica gel aluminum plates (Kieselgel 60, F254) and was visualized using a UV lamp at 254 nm. Dry-column flash chromatography was carried out using Merck silica gel 60H. Nuclear Magnetic Resonance spectra were obtained in CDCl3, using a Jeol JNM-ECS400 spectrometer (JEOL Ltd., Akishima, Japan) (400 MHz). The high-resolution mass spectrum was recorded using the Thermo Scientific TSQ Vantage Triple State Quadrupole spectrometer (Thermo Fischer Scientific, San Jose, CA, USA), while the infrared spectrum was obtained using the Shimadzu 8400 S FTIR (Shimadzu, Kyoto, Japan) spectrophotometer.
3.1. Synthesis of Isoamyl (E)-3-(3,4-Dimethoxyphenyl)acrylate (1)
A solution of 3,4-dimethoxycinnamic acid (0.31 g, 1.50 mmol), DMAP (0.018 g, 0.15 mmol), MNBA (0.62 g, 1.80 mmol) and triethylamine (0.46 mL, 3.30 mmol) in anhydrous dichloromethane (15 mL) was stirred for 10 min at room temperature. Isoamyl alcohol (0.16 mL; 1.50 mmol) was then added, and the solution was stirred for an additional 190 min. The reaction was monitored by means of thin layer chromatography with ethyl acetate/n-hexane (1/2) as the eluent. The solvent was removed under reduced pressure, and the crude product was purified by means of dry-column flash chromatography with ethyl acetate/n-hexane (1/20) as the eluent to obtain isoamyl (E)-3-(3,4-dimethoxyphenyl)acrylate (1) in the form of a yellowish liquid (0.40 g, 95% yield). Rf = 0.6 (ethyl acetate/n-hexane,1/2); 1H NMR (400 MHz, CDCl3) δ 0.95 (d, J = 6.8 Hz, 6H, CH3); 1.59 (q, J = 6.8 Hz, 2H, CH2CH2O); 1.72–1.74 (m, J = 6.8 Hz, 1H, CH(CH3)2); 3.91 (s, 6H, OCH3); 4.23 (t, J = 6.8 Hz, 2H, CH2CH2O); 6.30 (d, J = 16.0 Hz, 1H, CH=); 6.86 (d, J = 8.0 Hz, 1H, ArH); 7.05 (d, J = 2.0 Hz, 1H, ArH); 7.10 (dd, J = 2.0; 8.0 Hz, 1H, ArH); 7.62 (d, J = 16.0 Hz, 1H, CH=). 13C NMR (100 MHz, CDCl3) δ 22.59 (CH3), 25.18 CH(CH3)2, 37.55 (CH2CH2O), 55.95 (OCH3), 56.05 (OCH3), 63.19 (CH2CH2O), 109.59 (ArCH), 111.06 (ArCH), 116.05 (CH=), 122.68 (ArCH), 127.52 (ArC), 144.57 (CH=), 149.26 (ArC), 151.12 (ArC), 167.43 (C=O). FTIR (KBr) v (cm−1) 1702 (C=O ester), 1157 (C-O ester). HRESIMS m/z (pos): 301.1418 C18H18O2Na (calcd. 301.1416) (Supplementary Materials).
3.2. In Vitro Sun Protection Factor Determination
Determination of sun protection factor (SPF) value of isoamyl (E)-3-(3,4-dimethoxyphenyl)acrylate (1) was carried out by means of the ultraviolet-visible spectrophotometry method described by Mansur et al. [15]. Solution of compound (1) was prepared at a concentration of 1 mg.mL−1 in 96% ethanol. The absorbance was then measured at 290–320 nm. SPF values were determined using Equation (1) as follows:
where CF is the correction factor (10, EE(λ)) is the erythemogenic effect of wavelength radiation (λ), I(λ) is the intensity of solar radiation in the wavelength (λ), and Abs(λ) is the spectrophotometry reading of the absorbance of sunscreen solution in the wavelength (λ).
4. Conclusions
Isoamyl (E)-3-(3,4-dimethoxyphenyl)acrylate (1) was successfully synthesized through MNBA/DMAP-mediated esterification in 95% yield. Compound (1) obtained an SPF value of 37.10 ± 0.03 which falls in the high category indicating that it is a sunscreen agent with high protection.
Supplementary Materials
The following supporting information can be downloaded online, Figure S1: IR spectrum of compound (1); Figure S2: 1H NMR spectrum of compound (1); Figure S3: 13C NMR spectrum of (1); Figure S4: High Resolution Mass spectrum of compound (1); Figure S5: Plausible mechanism for the formation of isoamyl (E)-3-(3,4-dimethoxyphenyl)acrylate (1).
Author Contributions
Conceptualization, M.S.; methodology, M.S. and E.P.; software, E.P.; validation, M.S. and L.A.; formal analysis, E.P.; investigation, E.P.; resources, M.S.; data curation, M.S.; writing—original draft preparation, E.P.; writing—review and editing, M.S. and L.A.; visualization, E.P.; supervision, M.S.; project administration, M.S.; funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Ministry of Education and Culture, Research, and Technology of Indonesia Republic (Penelitian Fundamental grant number 1945/PKS/ITS/2023).
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.
Acknowledgments
The authors acknowledge Ministry of Education and Culture, Research, and Technology of Indonesia Republic for supporting fund.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Steele, J.H.; Bozor, M.X.; Boyce, G.R. Transmutation of Scent: An Evaluation of the Synthesis of Methyl Cinnamate, a Commercial Fragrance, via a Fischer Esterification for the Second-Year Organic Laboratory. J. Chem. Educ. 2020, 97, 4127–4132. [Google Scholar] [CrossRef]
- Wong, N.G.K.; Dessent, C.E.H. Illuminating the Effect of the Local Environment on the Performance of Organic Sunscreens: Insights from Laser Spectroscopy of Isolated Molecules and Complexes. Front. Chem. 2022, 9, 812098. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Jiang, Y.; Zhang, H.; Huang, W.; Xie, Y.; Deng, C.; Xu, H.; Song, H.; Xu, H. Design, synthesis of Cinnamyl-paeonol derivatives with 1, 3-Dioxypropyl as link arm and screening of tyrosinase inhibition activity in vitro. Bioorg. Chem. 2021, 106, 104512. [Google Scholar] [CrossRef] [PubMed]
- Park, P.J.; Cho, E.G. Kojyl Cinnamate Ester Derivatives Increase Adiponectin Expression and Stimulate Adiponectin-Induced Hair Growth Factors in Human Dermal Papilla Cells. Int. J. Mol. Sci. 2019, 20, 1859. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yoo, J. History of sunscreen: An updated view. J. Cosmet. Dermatol. 2021, 20, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Martincigh, B.S.; Ollengo, M.A. The Photostabilizing Effect of Grape Seed Extract on Three Common Sunscreen Absorbers. J. Photochem. Photobiol. 2016, 92, 870–884. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Photoprotection and Skin Pigmentation: Melanin-Related Molecules and Some Other New Agents Obtained from Natural Sources. Molecules 2020, 25, 1537. [Google Scholar] [CrossRef] [PubMed]
- Kuitunen, M.-L.; Cecilia Altamirano, J.; Siegenthaler, P.; Hannele Taure, T.; Antero Häkkinen, V.; Sinikka Vanninen, P. Derivatization and rapid GC-MS screening of chlorides relevant to the chemical weapons convention in organic liquid samples. Anal. Methods 2020, 12, 2527–2535. [Google Scholar] [CrossRef] [PubMed]
- Amini, N.; Handayani, S.; Hudiyono, S. Toxicity test using brine shrimp lethality test (BSLT) and antioxidant assay of ricinoleic acid-based ester conjugate synthesized by Steglich esterification. AIP Conf. Proc. 2022, 2638, 070012. [Google Scholar] [CrossRef]
- Han, S.Y.; Kim, Y.A. Recent Development of Peptide Coupling Reagents in Organic Synthesis. Tetrahedron 2004, 60, 2447–2467. [Google Scholar] [CrossRef]
- Jordan, A.; Whymark, K.D.; Sydenham, J.; Sneddon, H.F. A Solvent-Reagent Selection Guide for Steglich-Type Esterification of Carboxylic Acids. Green. Chem. 2021, 23, 6405–6413. [Google Scholar] [CrossRef]
- Shiina, I.; Nakata, K. The first asymmetric esterification of free carboxylic acids with racemic alcohols using benzoic anhydrides and tetramisole derivatives: An application to the kinetic resolution of secondary benzylic alcohols. Tetrahedron Lett. 2007, 48, 8314–8317. [Google Scholar] [CrossRef]
- Manaia, E.B.; Kaminski, R.C.K.; Corrêa, M.A.; Chiavacci, L.A. Inorganic UV filters. Braz. J. Pharm. Sci. 2013, 49, 201–209. [Google Scholar] [CrossRef]
- Facts about Sunscreen. Available online: https://www.fda.gov/news-events/rumor-control/facts-about-sunscreen (accessed on 5 July 2023).
- Mansur, J.d.S.; Breder, M.N.R.; Mansur, M.C.d.A. Determinação do fator de proteção solar por espectrofotometria. An. Bras. Dermatol. 1986, 61, 121–124. [Google Scholar]
- Gunia-Krzyżak, A.; Słoczyńska, K.; Popiół, J.; Koczurkiewicz, P.; Marona, H.; Pękala, E. Cinnamic acid derivatives in cosmetics: Current use and future prospects. Int. J. Cosmet. Sci. 2018, 40, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, C.; Kosmadaki, M.G.; Stratigos, A.J.; Katsambas, A.D. Sunscreens—What’s important to know. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Kiss, B.; Bíró, T.; Czifra, G.; Tóth, B.; Kertész, Z.; Szikszai, Z.; Kiss, A.Z.; Juhászl, I.; Zouboulis, C.C.; Hunyadi, J. Investigation of micronized titanium dioxide penetration in human skin xenografts and its effect on cellular functions of human skin-derived cells. Exp. Dermatol. 2008, 17, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, S.; Harabuchi, Y.; Inokuchi, Y.; Maeda, S.; Ehara, M.; Yamazaki, K.; Ebata, T. Substitution effect on the nonradiative decay and trans → cis photoisomerization route: A guideline to develop efficient cinnamate-based sunscreens. Phys. Chem. Chem. Phys. 2021, 23, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Dalton, J.; Richings, G.W.; Woolley, J.M.; Abiola, T.T.; Habershon, S.; Stavros, V.G. Experimental and Computational Analysis of Para-Hydroxy Methylcinnamate following Photoexcitation. Molecules 2021, 26, 7621. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.P.; Li, C.X.; Xie, B.B.; Cui, G. Photoprotection Mechanism of p-Methoxy Methylcinnamate: A CASPT2 Study. J. Phys. Chem. 2015, 119, 11488–11497. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).