4,4′,4″-(Benzene-1,3,5-triyltris(ethyne-2,1-diyl))tris(1-methylpyridin-1-ium) Iodide
Abstract
:1. Introduction
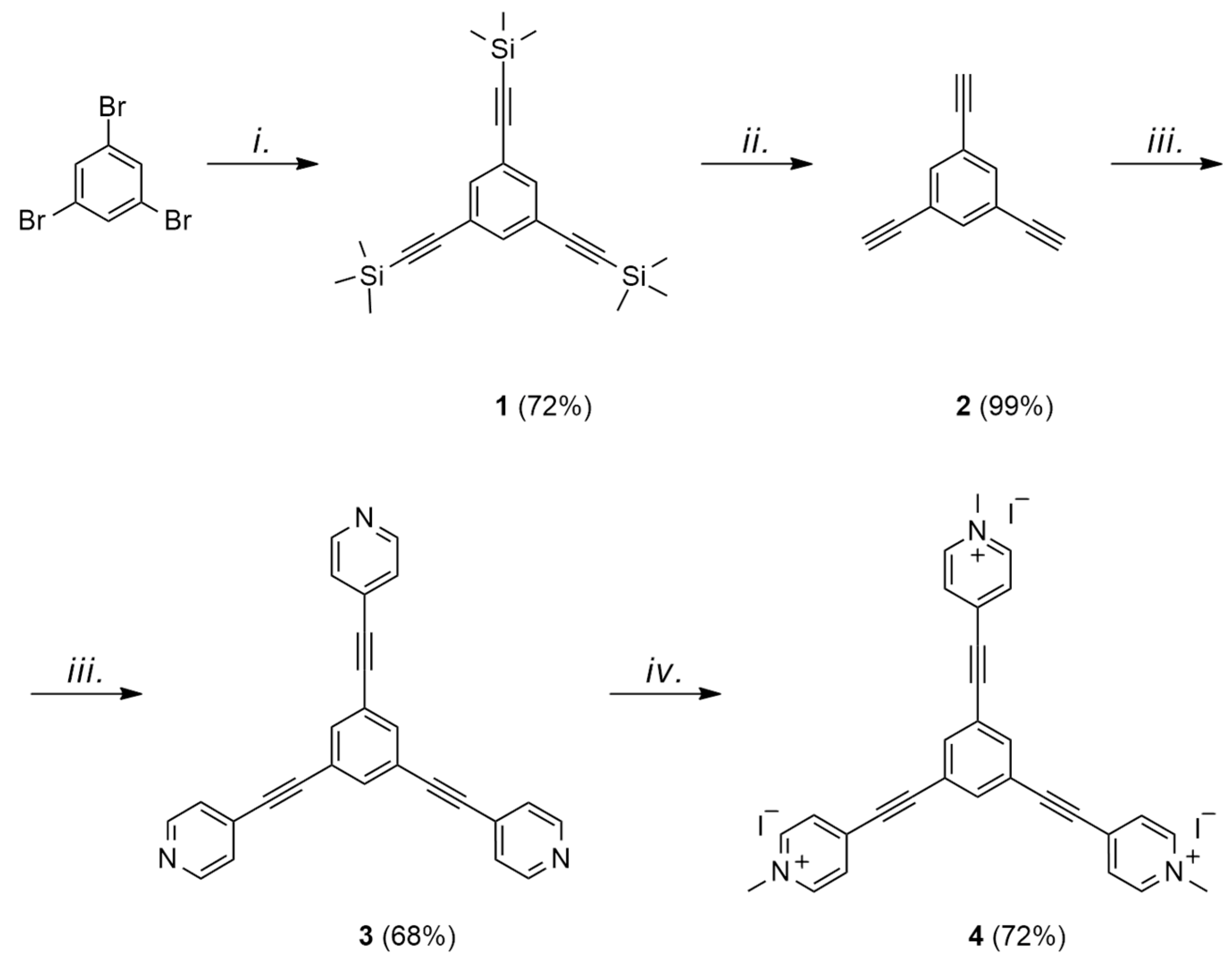
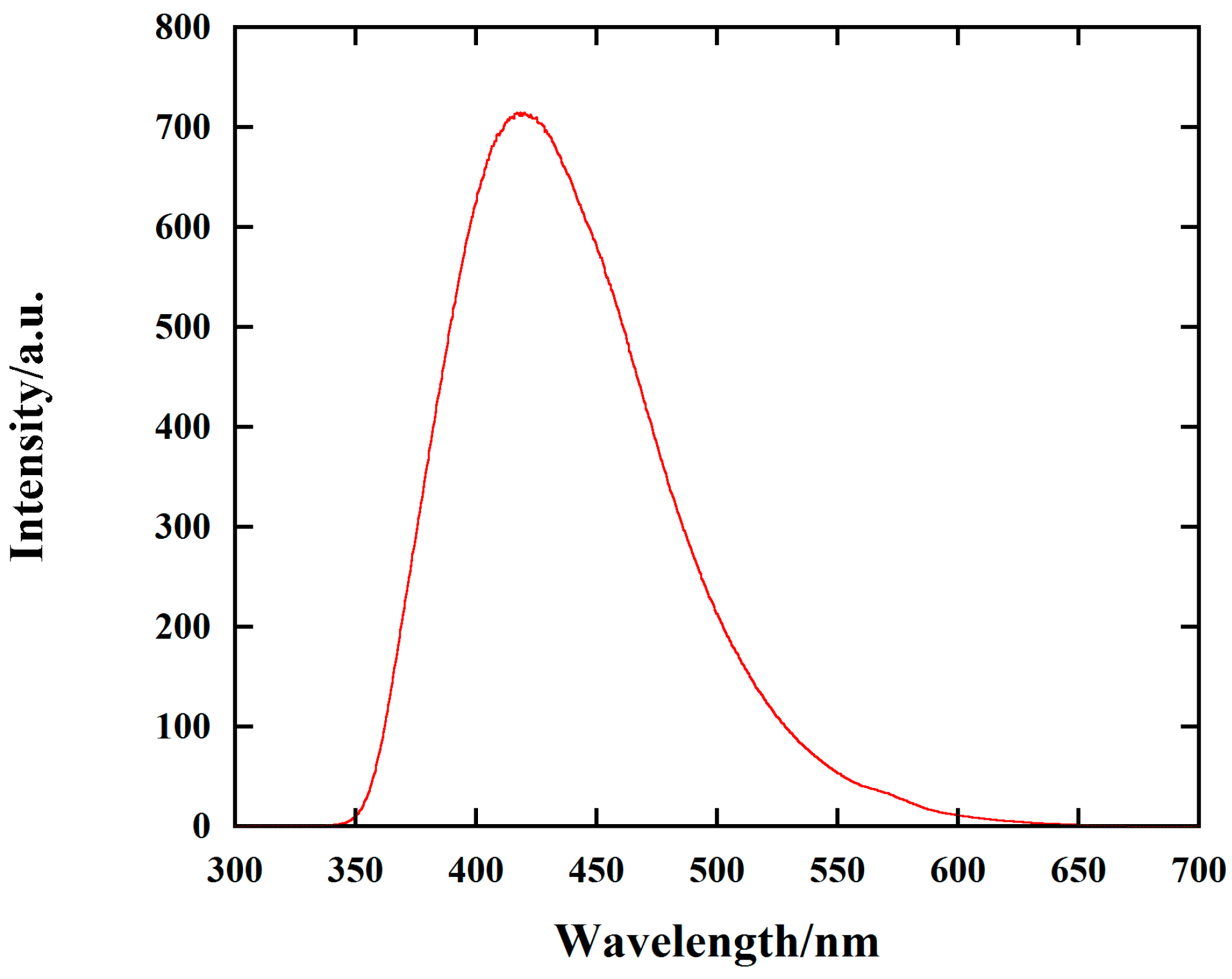
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Michaelis, L.; Hill, E.S. The viologen indicators. J. Gen. Physiol. 1933, 16, 859–873. [Google Scholar] [CrossRef]
- Moss, G.P.; Smith, P.A.S.; Tavernier, D. Glossary of class names of organic compounds and reactivity intermediates based on structure (IUPAC Recommendations 1995). Pure Appl. Chem. 1995, 67, 1307–1375. [Google Scholar] [CrossRef]
- Ross, J.H.; Krieger, R.I. Synthesis and properties of paraquat (methyl viologen) and other herbicidal alkyl homologues. J. Agric. Food Chem. 1980, 28, 1026–1031. [Google Scholar] [CrossRef]
- Sartori, F.; Vidrio, E. Environmental fate and ecotoxicology of paraquat: A California perspective. Toxicol. Environ. Chem. 2018, 100, 479–517. [Google Scholar] [CrossRef]
- Tsai, W.-T. A review on environmental exposure and health risks of herbicide paraquat. Toxicol. Environ. Chem. 2013, 95, 197–206. [Google Scholar] [CrossRef]
- Stuart, A.M.; Merfield, C.N.; Horgan, F.G.; Willis, S.; Watts, M.A.; Ramírez-Muñoz, F.; Sánchez, U.J.; Utyasheva, L.; Eddleston, M.; Davis, M.L.; et al. Agriculture without paraquat is feasible without loss of productivity—Lessons learned from phasing out a highly hazardous herbicide. Environ. Sci. Pollut. Res. 2023, 30, 16984–17008. [Google Scholar] [CrossRef]
- Striepe, L.; Baumgartner, T. Viologens and Their Application as Functional Materials. Chem. Eur. J. 2017, 23, 16924–16940. [Google Scholar] [CrossRef]
- Škorjanc, T.; Shetty, D.; Olson, M.A.; Trabolsi, A. Design Strategies and Redox-Dependent Applications of Insoluble Viologen-Based Covalent Organic Polymers. ACS Appl. Mater. Interfaces 2019, 11, 6705–6716. [Google Scholar] [CrossRef]
- Li, X.; Yang, J.; Yang, Y.-W. Recent advances of stimuli-responsive viologen-based nanocomposites. Mater. Chem. Front. 2023, 7, 1463–1481. [Google Scholar] [CrossRef]
- Kanagaraj, M.; Velayutham, D.; Suryanarayanan, V.; Kathiresan, M.; Ho, K.-C. Viologen-based electrochromic materials and devices. J. Mater. Chem. C 2019, 7, 4622–4637. [Google Scholar]
- Shah, K.W.; Wang, S.-X.; Soo, D.X.Y.; Xu, J. Viologen-Based Electrochromic Materials: From Small Molecules, Polymers and Composites to Their Applications. Polymers 2019, 11, 1839. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Guo, S.; Ma, D.; Wang, J. An overview of electrochromic devices with electrolytes containing viologens. Sol. Energy Mater. Sol. Cells 2023, 254, 112270. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, G.; Chen, Q.; Zheng, J.; Xu, C. Electrochromism and electrochromic devices of new extended viologen derivatives with various substituent benzene. Sol. Energy Mater. Sol. Cells 2020, 208, 110413. [Google Scholar] [CrossRef]
- Dashitsyrenova, D.D.; Adonin, S.A.; Gorokh, I.D.; Kraevaya, O.A.; Pavlova, A.V.; Abramov, P.A.; Frolova, L.A.; Sokolov, M.N.; Fedin, V.P.; Troshin, P.A. Memory devices based on novel alkyl viologen halobismuthate(III) complexes. Chem. Commun. 2020, 56, 9162–9165. [Google Scholar] [CrossRef]
- Zhang, L.; Qiu, Y.; Liu, W.G.; Chen, H.; Shen, D.; Song, B.; Cai, K.; Wu, H.; Jiao, Y.; Feng, Y.; et al. An electric molecular motor. Nature 2023, 613, 280–286. [Google Scholar] [CrossRef]
- Kandpal, S.; Ghosh, T.; Rani, C.; Tanwar, M.; Sharma, M.; Rani, S.; Pathak, D.K.; Bathia, R.; Sameera, I.; Jayabalan, J.; et al. Bifunctional Application of Viologen-MoS2-CNT/Polythiophene Device as Electrochromic Diode and Half-Wave Rectifier. ACS Mater. Au 2022, 2, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, Y.; Li, X.; Zhang, W.; Ma, H. Trace SO2 Gas Capture in Stable 3D Viologen Ionic Porous Organic Framework Microsphere. ACS Appl. Mater. Interfaces 2023, 15, 30312–30319. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, J.; Tan, A.; Fu, Z. A Highly Efficient Near-Infrared-Activated Photocatalyst Based on an Electron-Deficient Copper-Viologen-Polyoxometalate Framework with a Copper {Cu3} Cluster Decorated Phosphotungstate as a Building Block. Cryst. Growth Des. 2019, 19, 6845–6849. [Google Scholar] [CrossRef]
- Sui, Q.; Li, R.; Zhang, Y.; Huang, M.; Wang, T.; Yang, M.; Cao, M.; Hong, X.; Li, B. High-efficient C3N4-viologen charge transfer systems for promoting photocatalytic H2 evolution through band engineering. Int. J. Hydrogen Energy 2023, 48, 16330–16340. [Google Scholar] [CrossRef]
- Ling, Z.; Nugraha, M.I.; Hadmojo, W.T.; Lin, Y.; Jeong, S.Y.; Yengel, E.; Faber, H.; Tang, H.; Laquai, F.; Emwas, A.-H.; et al. Over 19% Efficiency in Ternary Organic Solar Cells Enabled by n-Type Dopants. ACS Energy Lett. 2023, 8, 4104–4112. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Zuo, P.; Chen, Q.; Tang, G.; Sun, P.; Yang, Z.; Xu, T. Screening Viologen Derivatives for Neutral Aqueous Organic Redox Flow Batteries. ChemSusChem 2020, 13, 2245–2249. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zheng, C.; Wang, L.; Lu, C.; Zhang, B.; Chen, Y.; Li, M.; Zhai, G.; Zhuang, X. Viologen-inspired functional materials: Synthetic strategies and applications. J. Mater. Chem. A 2019, 7, 23337–23360. [Google Scholar] [CrossRef]
- Das, G.; Skorjanc, T.; Sharma, S.K.; Gándara, F.; Lusi, M.; Rao, D.S.S.; Vimala, S.; Prasad, S.K.; Raya, J.; Han, D.S.; et al. Viologen-Based Conjugated Covalent Organic Networks via Zincke Reaction. J. Am. Chem. Soc. 2017, 139, 9558–9565. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Kähkönen, A.; Damlin, P.; Ääritalo, T.; Kankare, J.; Kvarnström, C. Electrochemical synthesis and characterization of branched viologen derivatives. Electrochim. Acta 2015, 154, 361–369. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kärnbratt, J.; Chang, M.H.; Herz, L.M.; Albinsson, B.; Anderson, H.L. Enhanced π conjugation around a porphyrin[6] nanoring. Angew. Chem. Int. Ed. Engl. 2008, 47, 4993–4996. [Google Scholar] [CrossRef] [PubMed]
- Natali, M.; Luisa, A.; Iengo, E.; Scandola, F. Efficient photocatalytic hydrogen generation from water by a cationic cobalt(II) porphyrin. Chem. Commun. 2014, 50, 1842–1844. [Google Scholar] [CrossRef]
- Gao, P.; Pan, Y.; Han, H.; Gu, Z.; Chen, H.; Wu, Z.; Liu, H.; Peng, S.; Zhang, X.-P.; Zhang, R.; et al. Molecular engineering of π-extended viologens consisting of thiophene-based bridges for electrochromic devices. J. Mol. Struct. 2023, 1288, 135769. [Google Scholar] [CrossRef]
- Kanwal, I.; Mujahid, A.; Rasool, N.; Rizwan, K.; Malik, A.; Ahmad, G.; Shah, S.A.A.; Rashid, U.; Nasir, N.M. Palladium and Copper Catalyzed Sonogashira cross Coupling an Excellent Methodology for C-C Bond Formation over 17 Years: A Review. Catalysts 2020, 10, 443. [Google Scholar] [CrossRef]
- Yuan, K.; Zhuang, X.; Fu, H.; Brunklaus, G.; Forster, M.; Chen, Y.; Feng, X.; Scherf, U. Two-Dimensional Core-Shelled Porous Hybrids as Highly Efficient Catalysts for Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2016, 55, 6858–6863. [Google Scholar] [CrossRef]
- Anderson, H.L.; Walter, C.J.; Vidal-Ferran, A.; Hay, R.A.; Lowden, P.A.; Sanders, J.K.M. Octatetrayne-linked porphyrins: ‘stretched’ cyclic dimers and trimers with very spacious cavities. J. Chem. Soc. Perkin Trans. 1995, 1, 2275–2279. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romagnoli, L.; D’Annibale, A.; Latini, A. 4,4′,4″-(Benzene-1,3,5-triyltris(ethyne-2,1-diyl))tris(1-methylpyridin-1-ium) Iodide. Molbank 2023, 2023, M1742. https://doi.org/10.3390/M1742
Romagnoli L, D’Annibale A, Latini A. 4,4′,4″-(Benzene-1,3,5-triyltris(ethyne-2,1-diyl))tris(1-methylpyridin-1-ium) Iodide. Molbank. 2023; 2023(4):M1742. https://doi.org/10.3390/M1742
Chicago/Turabian StyleRomagnoli, Lorenza, Andrea D’Annibale, and Alessandro Latini. 2023. "4,4′,4″-(Benzene-1,3,5-triyltris(ethyne-2,1-diyl))tris(1-methylpyridin-1-ium) Iodide" Molbank 2023, no. 4: M1742. https://doi.org/10.3390/M1742
APA StyleRomagnoli, L., D’Annibale, A., & Latini, A. (2023). 4,4′,4″-(Benzene-1,3,5-triyltris(ethyne-2,1-diyl))tris(1-methylpyridin-1-ium) Iodide. Molbank, 2023(4), M1742. https://doi.org/10.3390/M1742





