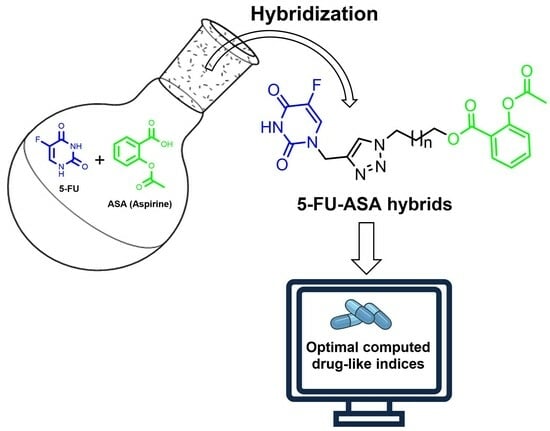
Synthesis and In Silico Drug-Likeness Modeling of 5-FU/ASA Hybrids
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. In Silico Pharmacokinetic Analysis of Conjugates 4a–h
3. Materials and Methods
3.1. Chemical Synthesis
General Procedure for the Synthesis of 5-FU-ASA Hybrids 4a–4h
3.2. Drug-Likeness Modelling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meunier, B. Hybrid molecules with a dual mode of action: Dream or reality? Acc. Chem. Res. 2008, 41, 69–77. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Pedrosa, M.; Duarte da Cruz, R.M.; de Oliveira Viana, J.; de Moura, R.O.; Ishiki, H.M.; Barbosa Filho, J.M.; Diniz, M.F.; Scotti, M.T.; Scotti, L.; Bezerra Mendonca, F. Hybrid Compounds as Direct Multitarget Ligands: A Review. Curr. Top. Med. Chem. 2017, 17, 1044–1079. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. Available online: https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21660 (accessed on 1 June 2021). [CrossRef]
- Alqahtani, Z.; Jamali, F. Clinical outcomes of aspirin interaction with other non-steroidal anti-inflammatory drugs: A systematic review. J. Pharm. Pharm. Sci. 2018, 21, 29854. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Yan, Y.; Chen, M.; Luo, G.; Hao, J.; Pan, J.; Hu, S.; Guo, P.; Li, W.; Wang, R.; et al. Aspirin enhances the sensitivity of colon cancer cells to cisplatin by abrogating the binding of NF-κB to the COX-2 promoter. Aging 2020, 12, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Saxena, M.; Rishi, N. An Overview of Recent Advances in Biomedical Applica-tions of Click Chemistry. Bioconjug. Chem. 2021, 32, 1455–1471. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiong, Y. Application of “Click” Chemistry in Biomedical Hydrogels. ACS Omega 2022, 7, 36918–36928. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar Yadav, A.; Mishra, V.; Kumar, D. Recent Advance-ments in Triazole-based Click Chemistry in Cancer Drug Discovery and Development. Syn. Open 2023, 7, 186–208. [Google Scholar]
- Moreno-Quintero, G.; Betancur-Zapata, E.; Herrera-Ramírez, A.; Cardona-Galeano, W. New Hybrid Scaffolds Based on 5-FU/Curcumin: Synthesis, Cytotoxic, Antiproliferative and Pro-Apoptotic Effect. Pharmaceutics 2023, 15, 1221. [Google Scholar] [CrossRef]
- Moreno-Quintero, G.; Castrillón-Lopez, W.; Herrera-Ramirez, A.; Yepes-Pérez, A.F.; Quintero-Saumeth, J.; Cardona-Galeano, W. Synthesis and Chemopreventive Potential of 5-FU/Genistein Hybrids on Colorectal Cancer Cells. Pharmaceuticals 2022, 15, 1299. [Google Scholar] [CrossRef]
- Gómez-R, L.; Moreno-Q, G.; Herrera-R, A.; Castrillón-L, W.; Yepes, A.F.; Cardona, G.W. New Hybrid Scaffolds Based on ASA/Genistein: Synthesis, Cytotoxic Effect, Molecular Docking, Drug-likeness and in silico ADME/tox Modeling. J. App. Pharm. Sci. 2022, 12, 15–30. [Google Scholar]
- Belkharchach, S.; Ighachane, H.; Lachgar, A.; Ait Ali, M.; Lazrek, H.B. Efficient and selective catalytic N-Alkylation of pyrimidine by ammonium Sulfate@Hydro-thermal carbone under eco-friendly conditions. J. Chem. Sci. 2020, 132, 138. [Google Scholar] [CrossRef]
- Kuan, H.; Xie, Y.; Guo, Y.; Gianoncelli, A.; Ribaudo, G.; Coghi, P. (2R, 4S, 5S) 1-(4-(4-(((7-Chloroquinolin-4-yl)amino)methyl)-1H-1,2,3-triazol-1-yl)-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-methylpyrimidine-2,4(1H,3H)-dione. Molbank 2023, 2023, M1681. [Google Scholar] [CrossRef]
- Karabulut, H.R.F.; Karatavuk, A.O.; Ozyildirim, H.; Doğanlar, O.; Doğanlar, Z.B. Synthesis of novel dimeric compounds containing triazole using click method and their selective antiproliferative and proapoptotic potential via mitochondrial apoptosis signaling. Med. Chem. Res. 2020, 29, 643–655. [Google Scholar] [CrossRef]
- Iwata, H. Application of in Silico Technologies for Drug Target Discovery and Pharmacokinetic Analysis. Chem. Pharm. Bull. 2023, 71, 398–405. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Ditzinger, F.; Price, D.J.; Ilie, A.R.; Köhl, N.J.; Jankovic, S.; Tsakiridou, G.; Aleandri, S.; Kalantzi, L.; Holm, R.; Nair, A.; et al. Lipophilicity and hydrophobicity considerations in bio-enabling oral formulations approaches—A PEARRL review. J. Pharm. Pharmacol. 2019, 71, 464–482. [Google Scholar] [CrossRef]
- Ertl, P.; Rohdem, B.; Selzer, P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef]
- Baell, J.B.; Nissink, J.W.M. Seven Year Itch: Pan-Assay Interference Compounds (PAINS) in 2017-Utility and Limitations. ACS Chem. Biol. 2018, 13, 36–44. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]


| Comp. (n) | MW a | LogPo/w b | R.B. c | n-ON d | n-OHNH e | TPSA f | LRFV g | PAINS h |
|---|---|---|---|---|---|---|---|---|
| 4a | 417.35 | 0.61 | 9 | 10 | 1 | 138.19 | 0 | 0 |
| 4b | 431.38 | 0.88 | 10 | 10 | 1 | 138.19 | 0 | 0 |
| 4c | 445.41 | 1.15 | 11 | 10 | 1 | 138.19 | 0 | 0 |
| 4d | 459.43 | 1.66 | 12 | 10 | 1 | 138.19 | 0 | 0 |
| 4e | 473.46 | 2.16 | 13 | 10 | 1 | 138.19 | 0 | 0 |
| 4f | 501.51 | 3.16 | 15 | 10 | 1 | 138.19 | 1 | 0 |
| 4g | 515.54 | 3.68 | 16 | 10 | 1 | 138.19 | 1 | 0 |
| 4h | 557.62 | 5.19 | 19 | 10 | 1 | 138.19 | 1 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castrillón-López, W.; Yepes, A.F.; Cardona-Galeano, W. Synthesis and In Silico Drug-Likeness Modeling of 5-FU/ASA Hybrids. Molbank 2023, 2023, M1745. https://doi.org/10.3390/M1745
Castrillón-López W, Yepes AF, Cardona-Galeano W. Synthesis and In Silico Drug-Likeness Modeling of 5-FU/ASA Hybrids. Molbank. 2023; 2023(4):M1745. https://doi.org/10.3390/M1745
Chicago/Turabian StyleCastrillón-López, Wilson, Andrés F. Yepes, and Wilson Cardona-Galeano. 2023. "Synthesis and In Silico Drug-Likeness Modeling of 5-FU/ASA Hybrids" Molbank 2023, no. 4: M1745. https://doi.org/10.3390/M1745
APA StyleCastrillón-López, W., Yepes, A. F., & Cardona-Galeano, W. (2023). Synthesis and In Silico Drug-Likeness Modeling of 5-FU/ASA Hybrids. Molbank, 2023(4), M1745. https://doi.org/10.3390/M1745