5,5′-Bis[9-(2-ethylhexyl)-9H-carbazol-3-yl]-4,4′-diphenyl-2,2′-bithiazole
Abstract
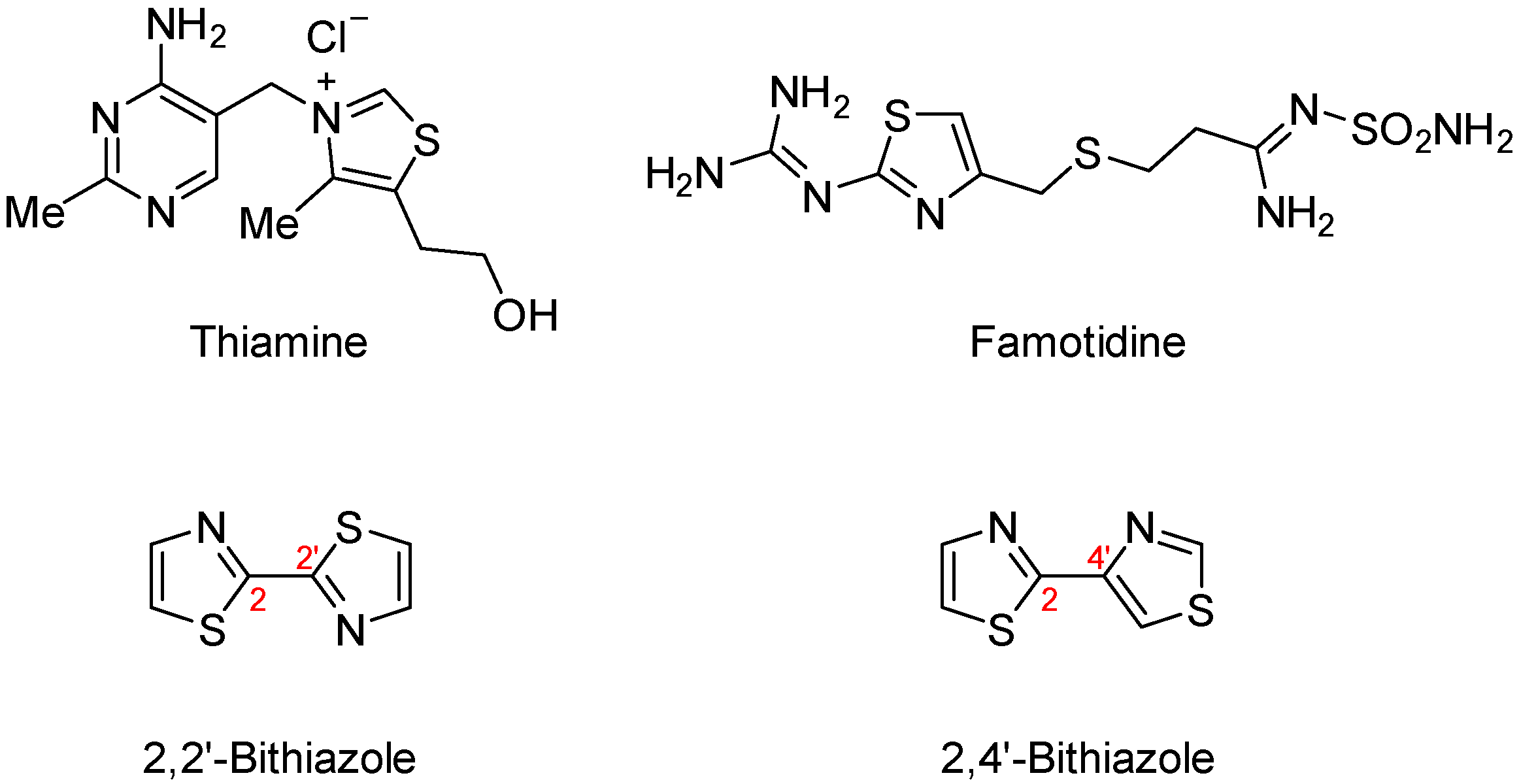
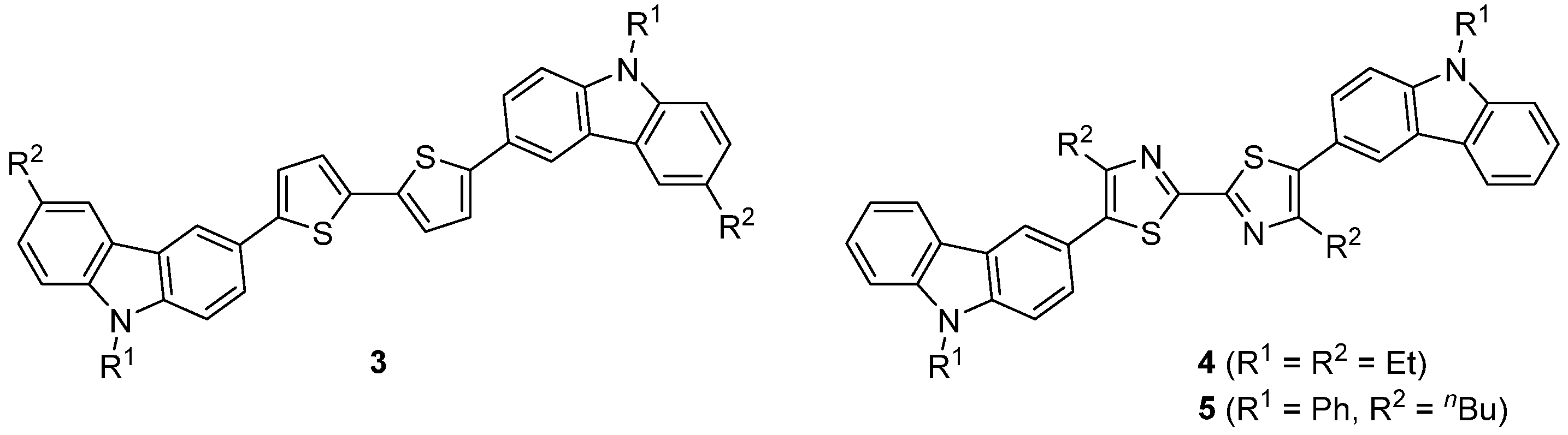
:1. Introduction
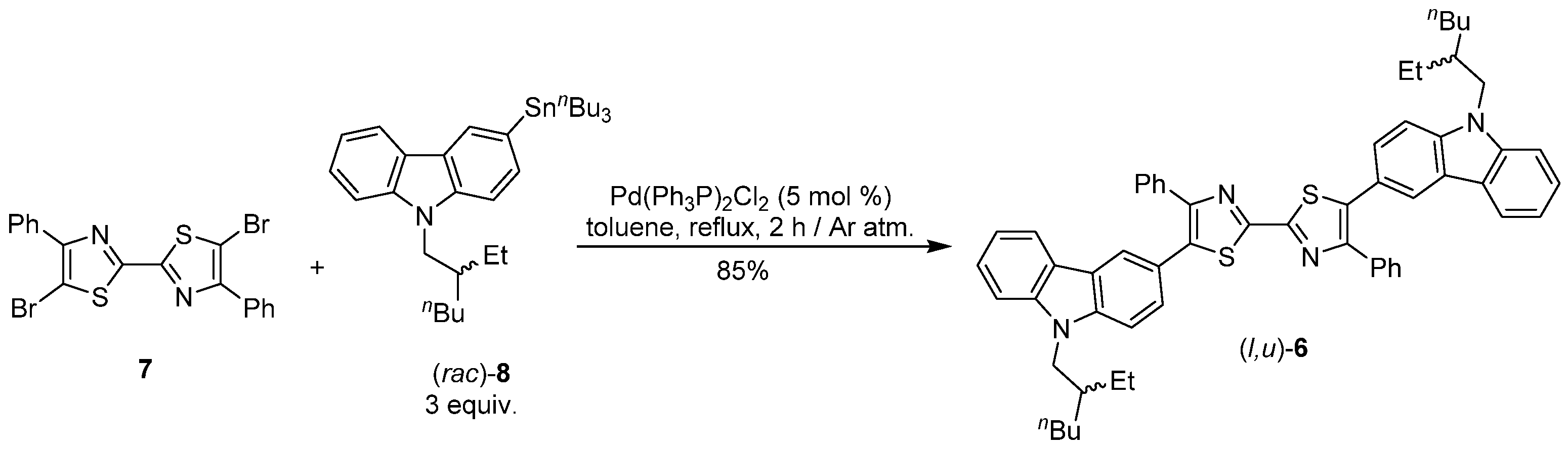
2. Results and Discussion
3. Materials and Methods
5,5′-Bis [9-(2-ethylhexyl)-9H-carbazol-3-yl]-4,4′-diphenyl-2,2′-bithiazole (6)
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chotera-Ouda, A.; Wróblewska, A.; Tokarz, P.; Stevens, C.V. Thiazoles. In Comprehensive Heterocyclic Chemistry IV; Koutentis, P.A., StC Black, D., Cossy, J., Stevens, C.V., Eds.; Elsevier: Oxford, UK, 2022; Volume 4, Chapter 4.06; pp. 530–623. [Google Scholar] [CrossRef]
- Chen, B.; Heal, W. Thiazoles. In Comprehensive Heterocyclic Chemistry III; Zhdankin, V.V., Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Oxford, UK, 2008; Volume 4, Chapter 4.06; pp. 635–754. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Ioannidou, H.A. Thiazoles. In Science of Synthesis Knowledge Updates 2010/1; Schaumann, E., Ed.; Thieme Chemistry: Stuttgart, Germany, 2010; Volume 11, Product Class 17; pp. 267–391. [Google Scholar]
- Fattal-Valevski, A. Thiamine (Vitamin B1). J. Evid. Based Complement. Altern. Med. 2011, 16, 12–20. [Google Scholar] [CrossRef]
- Lea, A.P.; Faulds, D. Ritonavir. Drugs 1996, 52, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Takeda, M.; Fujihara, A.; Yashima, Y. General pharmacological properties of a new potent H2-blocker, famotidine (YM-11170). Pharmacometrics 1983, 26, 599–611. [Google Scholar]
- Umezawa, H.; Maeda, K.; Takeuchi, T.; Okami, Y. New antibiotics, bleomycin A and B. J. Antibiot. 1966, 19, 200–209. [Google Scholar]
- Ando, S.; Murakami, R.; Nishida, J.; Tada, H.; Inoue, Y.; Tokito, S.; Yamashita, Y. n-Type organic field-effect transistors with very high electron mobility based on thiazole oligomers with trifluoromethylphenyl Groups. J. Am. Chem. Soc. 2005, 127, 14996–14997. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, L.; Yang, D.; Ma, S.; Zhou, X.; Zhang, J.; Tu, G.; Li, C. A non-fullerene acceptor with all “A” units realizing high open-circuit voltage solution-processed organic photovoltaics. J. Mater. Chem. A 2014, 2, 2657–2662. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Michaelidou, S.S.; White, A.J.P.; Koutentis, P.A. Transformation of 2-(4-chloro-5H-1,2,3-dithiazol-5-ylideneamino)-6-ethoxy-4-phenylpyridine-3,5-dicarbonitrile into 4-aminopyrido[2,3-d]pyrimidines and 2-(pyrid-2-yl)guanidines. Tetrahedron 2015, 71, 1799–1807. [Google Scholar] [CrossRef]
- Koyioni, M.; Koutentis, P.A. Synthesis of 5,5′-Diarylimino Quinoidal 2,2′-Bithiazoles. Org. Lett. 2017, 19, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Hermerschmidt, F.; Kalogirou, A.S.; Min, J.; Zissimou, G.A.; Tuladhar, S.M.; Ameri, T.; Faber, H.; Itskos, G.; Choulis, S.A.; Anthopoulos, T.D.; et al. 4H-1,2,6-Thiadiazin-4-one-containing small molecule donors and additive effects on their performance in solution-processed organic solar cells. J. Mater. Chem. C 2015, 3, 2358–2365. [Google Scholar] [CrossRef]
- Chochos, C.L.; Kalogirou, A.S.; Ye, T.; Tatsi, E.; Katsouras, A.; Zissimou, G.A.; Gregoriou, V.G.; Avgeropoulos, A.; Koutentis, P.A. 4H-1,2,6-Thiadiazine-containing donor–acceptor conjugated polymers: Synthesis, optoelectronic characterization and their use in organic solar cells. J. Mater. Chem. C 2018, 6, 3658–3667. [Google Scholar] [CrossRef]
- Inoue, M.; Hayashi, K. Material for Photoelectric Conversion Element for Imaging, and Photoelectric Conversion Element. WO2023/140173, 27 July 2023. [Google Scholar]
- Chang, G.-P.; Chuang, C.-N.; Lee, J.-Y.; Chang, Y.-S.; Leung, M.-K.; Hsieh, K.-H. Synthesis and characterization of graft polystyrenes with para-substituted π-conjugated oligo(carbazole) and oligo(carbazole-thiophene) moieties for organic field-effect transistors. Polymer 2013, 54, 3548–3555. [Google Scholar] [CrossRef]
- Chen, S.; Ma, T.; Du, X.; Mo, M.; Wang, Z.; Cheng, X. D-A-D hexacatenar LCs containing bulky N-trialkoxylbenzyl carbazole caps with RGB emissions for full color palette and white LED applications. J. Mol. Liq. 2023, 373, 121239. [Google Scholar] [CrossRef]
- Aksoy, B.T.; Senocak, A.; Erol, I.; Saglam, M.F.; Kandemir, H.; Sengul, I.F.; Çoşut, B. Meso carbazole linked Bis-BODIPYs: Design, synthesis, structures and properties. Tetrahedron 2023, 147, 133666. [Google Scholar] [CrossRef]
- Findlater, M.; Swisher, N.S.; White, P.S. Synthesis and Structure of Boron–Bithiazole Complexes. Eur. J. Org. Chem. 2010, 2010, 5379–5382. [Google Scholar] [CrossRef]
- Sharma, G.D.; Shanap, T.S.; Patel, K.R.; El-Mansy, M.K. Photovoltaic properties of bulk heterojunction devices based on CuI-PVA as electron donor and PCBM and modified PCBM as electron acceptor. Mater. Sci.-Pol. 2012, 30, 10–16. [Google Scholar] [CrossRef]

| Eox (V) | λmax (nm) | Egopt (eV) | EHOMO (eV) | Ered (V) | ELUMO (eV) | Egechem (eV) |
|---|---|---|---|---|---|---|
| 0.74 | 472 | 2.63 | −5.84 | −1.42 | −3.68 | 2.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalogirou, A.S.; Koutentis, P.A. 5,5′-Bis[9-(2-ethylhexyl)-9H-carbazol-3-yl]-4,4′-diphenyl-2,2′-bithiazole. Molbank 2024, 2024, M1761. https://doi.org/10.3390/M1761
Kalogirou AS, Koutentis PA. 5,5′-Bis[9-(2-ethylhexyl)-9H-carbazol-3-yl]-4,4′-diphenyl-2,2′-bithiazole. Molbank. 2024; 2024(1):M1761. https://doi.org/10.3390/M1761
Chicago/Turabian StyleKalogirou, Andreas S., and Panayiotis A. Koutentis. 2024. "5,5′-Bis[9-(2-ethylhexyl)-9H-carbazol-3-yl]-4,4′-diphenyl-2,2′-bithiazole" Molbank 2024, no. 1: M1761. https://doi.org/10.3390/M1761







