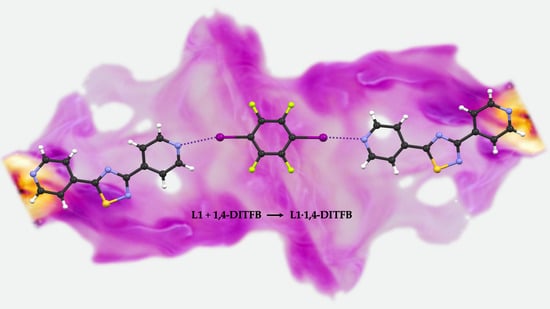
1,4-Diiodotetrafluorobenzene 3,5-di-(pyridin-4-yl)-1,2,4-thiadiazole <1/1>
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. General
3.2. Preparation of L1·1,4-DITFB (1)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Frija, L.M.T.; Pombeiro, A.J.L.; Kopylovich, M.N. Building 1,2,4-Thiadiazole: Ten Years of Progress. European J. Org. Chem. 2017, 2017, 2670–2682. [Google Scholar] [CrossRef]
- Iizawa, Y.; Okonogi, K.; Hayashi, R.; Iwahi, T.; Yamazaki, T.; Imada, A. Therapeutic Effect of Cefozopran (SCE-2787), a New Parenteral Cephalosporin, against Experimental Infections in Mice. Antimicrob. Agents Chemother. 1993, 37, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, R.; Baraldi, P.G.; Carrion, M.D.; Cruz-Lopez, O.; Preti, D.; Tabrizi, M.A.; Fruttarolo, F.; Heilmann, J.; Bermejo, J.; Estévez, F. Hybrid Molecules Containing Benzo[4,5]Imidazo[1,2-d][1,2,4]Thiadiazole and α-Bromoacryloyl Moieties as Potent Apoptosis Inducers on Human Myeloid Leukaemia Cells. Bioorg. Med. Chem. Lett. 2007, 17, 2844–2848. [Google Scholar] [CrossRef] [PubMed]
- Leung-Toung, R.; Tam, T.F.; Wodzinska, J.M.; Zhao, Y.; Lowrie, J.; Simpson, C.D.; Karimian, K.; Spino, M. 3-Substituted Imidazo[1,2-d][1,2,4]-Thiadiazoles: A Novel Class of Factor XIIIa Inhibitors. J. Med. Chem. 2005, 48, 2266–2269. [Google Scholar] [CrossRef] [PubMed]
- Perlovich, G.L.; Proshin, A.N.; Volkova, T.V.; Petrova, L.N.; Bachurin, S.O. Novel 1,2,4-Thiadiazole Derivatives as Potent Neuroprotectors: Approach to Creation of Bioavailable Drugs. Mol. Pharm. 2012, 9, 2156–2167. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Alonso, M.; Castro, A.; Pérez, C.; Moreno, F.J. First Non-ATP Competitive Glycogen Synthase Kinase 3 β (GSK-3β) Inhibitors: Thiadiazolidinones (TDZD) as Potential Drugs for the Treatment of Alzheimer’s Disease. J. Med. Chem. 2002, 45, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Cashman, J.R.; Hanzlik, R.P. Oxidation and Other Reactions of Thiobenzamide Derivatives of Relevance to Their Hepatotoxicity. J. Org. Chem. 1982, 47, 4645–4650. [Google Scholar] [CrossRef]
- Castro, A.; Castaño, T.; Encinas, A.; Porcal, W.; Gil, C. Advances in the Synthesis and Recent Therapeutic Applications of 1,2,4-Thiadiazole Heterocycles. Bioorg. Med. Chem. 2006, 14, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, R.I.; Lewis, A.D.; King, J.A. Antitubercular Substances. IV. Thioamides. J. Am. Chem. Soc. 1955, 77, 4062–4066. [Google Scholar] [CrossRef]
- Aragoni, M.C.; Arca, M.; Coles, S.J.; Crespo Alonso, M.; Coles, S.L.; Davies, R.P.; Hursthouse, M.B.; Isaia, F.; Lai, R.; Lippolis, V. Coordination Polymers and Polygons Using Di-Pyridyl-Thiadiazole Spacers and Substituted Phosphorodithioato Ni II Complexes: Potential and Limitations for Inorganic Crystal Engineering. CrystEngComm 2016, 18, 5620–5629. [Google Scholar] [CrossRef]
- Podda, E.; Arca, M.; Coles, S.J.; Crespo Alonso, M.; Isaia, F.; Pintus, A.; Lippolis, V.; Aragoni, M.C. Supramolecular Assemblies Tailored by Dipyridyl-1,2-4-Thiadiazoles: Influence of the Building Blocks in the Predictability of the Final Network. Supramol. Chem. 2020, 32, 267–275. [Google Scholar] [CrossRef]
- Aragoni, M.C.; Arca, M.; Caltagirone, C.; Castellano, C.; Demartin, F.; Garau, A.; Isaia, F.; Lippolis, V.; Montis, R.; Pintus, A. Cationic and Anionic 1D Chains Based on NH+⋯N Charge-Assisted Hydrogen Bonds in Bipyridyl Derivatives and Polyiodides. CrystEngComm 2012, 14, 5809–5823. [Google Scholar] [CrossRef]
- Peloquin, A.J.; McMillen, C.D.; Pennington, W.T. Isolation of Unique Heterocycles Formed from Pyridine-Thiocarboxamides as Diiodine, Iodide, or Polyiodide Salts. CrystEngComm 2022, 24, 6251–6261. [Google Scholar] [CrossRef]
- Wang, H.; Jin, W.J. Cocrystal Assembled by 1,4-Diiodotetrafluorobenzene and Phenothiazine Based on C - I...π/N/S Halogen Bond and Other Assisting Interactions. Acta Cryst. B 2017, 73, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Desijaru, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the halogen bond (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed]
- Aragoni, M.C.; Podda, E.; Chaudhary, S.; Bhasin, A.K.K.; Bhasin, K.K.; Coles, S.J.; Orton, J.B.; Isaia, F.; Lippolis, V.; Pintus, A.; et al. An Experimental and Theoretical Insight into I2/Br2 Oxidation of Bis(Pyridin-2-Yl)Diselane and Ditellane. Chem. Asian J. 2023, 18, e202300836. [Google Scholar] [CrossRef]
- Aragoni, M.C.; Podda, E.; Arca, M.; Pintus, A.; Lippolis, V.; Caltagirone, C.; Bartz, R.H.; Lenardão, E.J.; Perin, G.; Schumacher, R.F.; et al. An Unprecedented Non-Classical Polyinterhalogen Anion Made of [I2Cl]− and I2 at the 2-(p-Tolyl)Selenopheno[2,3-b]Pyridinium Cation Template. New J. Chem. 2022, 46, 21921–21929. [Google Scholar] [CrossRef]
- Yeo, C.I.; Tan, Y.S.; Kwong, H.C.; Lee, V.S.; Tiekink, E.R.T. I⋯N halogen bonding in 1: 1 co-crystals formed between 1,4-diiodotetrafluorobenzene and the isomeric n-pyridinealdazines (n = 2, 3 and 4): Assessment of supramolecular association and influence upon solid-state photoluminescence properties. CrystEngComm 2022, 24, 7579–7591. [Google Scholar] [CrossRef]
- Pigger, F.C.; Kapadia, P.P.; Swenson, D.C. Halogen bonded networks from pyridyl-substituted tetraarylethylenes and diiodotetrafluorobenzenes. CrystEngComm 2013, 15, 4386–4391. [Google Scholar] [CrossRef]
- Guardigli, C.; Liantonio, R.; Mele, M.L.; Metrangolo, P.; Resnati, G.; Pilati, T. Design and Synthesis of New Tectons for Halogen Bonding-driven Crystal Engineering. Supramol. Chem. 2003, 15, 177. [Google Scholar] [CrossRef]
- Vartanian, M.; Lucassen, A.C.B.; Shimon, L.J.W.; van der Boom, M.E. Cocrystallization of a Tripyridyl Donor with Perfluorinated Iodobenzene Derivatives: Formation of Different N⋯I Halogen Bonds Determining Network vs Plain Packing Crystals. Cryst. Growth Des. 2008, 8, 786. [Google Scholar] [CrossRef]
- Thalladi, V.R.; Weiss, H.C.; Bläser, D.; Boese, R.; Nangia, A.; Desiraju, G.R. C−H⋯F Interactions in the Crystal Structures of Some Fluorobenzenes. J. Am. Chem. Soc. 1998, 120, 8702–8710. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlisPro. Software System Version 1.171.39.8d; Rigaku Corporation, Oxford, UK, 2015.
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]



| C–I⋯N | dC–I (Å) | dI⋯N (Å) | αC–I⋯N (°) | ||
|---|---|---|---|---|---|
| a | C13–I1⋯N1 | 2.101(5) | 2.801(5) | 177.4(2) | |
| b | C16i–I2i⋯N4 | 2.092(5) | 2.947(4) | 168.3(2) | |
| C–H⋯F | dC–H (Å) | dH⋯F (Å) | dC⋯F (Å) | αC–H⋯F (°) | |
| c | C2–H2⋯F2ii | 0.95 | 2.450 | 3.307(6) | 150 |
| d | C4–H4⋯F3iii | 0.95 | 2.607 | 3.142(6) | 122 |
| e | C5–H5⋯F3iii | 0.95 | 2.505 | 3.111(6) | 116 |
| Symmetry codes: i = 2 + x, −1 + y, −1 + z; ii = 1 − x, 2 − y, 1 − z; iii = −x, 1 − y, 1 − z. | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podda, E.; Pintus, A.; Lippolis, V.; Isaia, F.; Slawin, A.M.Z.; Carpenter-Warren, C.L.; Woollins, J.D.; Aragoni, M.C. 1,4-Diiodotetrafluorobenzene 3,5-di-(pyridin-4-yl)-1,2,4-thiadiazole <1/1>. Molbank 2024, 2024, M1801. https://doi.org/10.3390/M1801
Podda E, Pintus A, Lippolis V, Isaia F, Slawin AMZ, Carpenter-Warren CL, Woollins JD, Aragoni MC. 1,4-Diiodotetrafluorobenzene 3,5-di-(pyridin-4-yl)-1,2,4-thiadiazole <1/1>. Molbank. 2024; 2024(2):M1801. https://doi.org/10.3390/M1801
Chicago/Turabian StylePodda, Enrico, Anna Pintus, Vito Lippolis, Francesco Isaia, Alexandra M. Z. Slawin, Cameron L. Carpenter-Warren, John Derek Woollins, and Maria Carla Aragoni. 2024. "1,4-Diiodotetrafluorobenzene 3,5-di-(pyridin-4-yl)-1,2,4-thiadiazole <1/1>" Molbank 2024, no. 2: M1801. https://doi.org/10.3390/M1801
APA StylePodda, E., Pintus, A., Lippolis, V., Isaia, F., Slawin, A. M. Z., Carpenter-Warren, C. L., Woollins, J. D., & Aragoni, M. C. (2024). 1,4-Diiodotetrafluorobenzene 3,5-di-(pyridin-4-yl)-1,2,4-thiadiazole <1/1>. Molbank, 2024(2), M1801. https://doi.org/10.3390/M1801