Dichloro-μ2,μ2-naphthalene-1,8-diyl-bis(N,N,N′,N′-tetramethylethylenediamino)tetracopper(I)
Abstract
1. Introduction
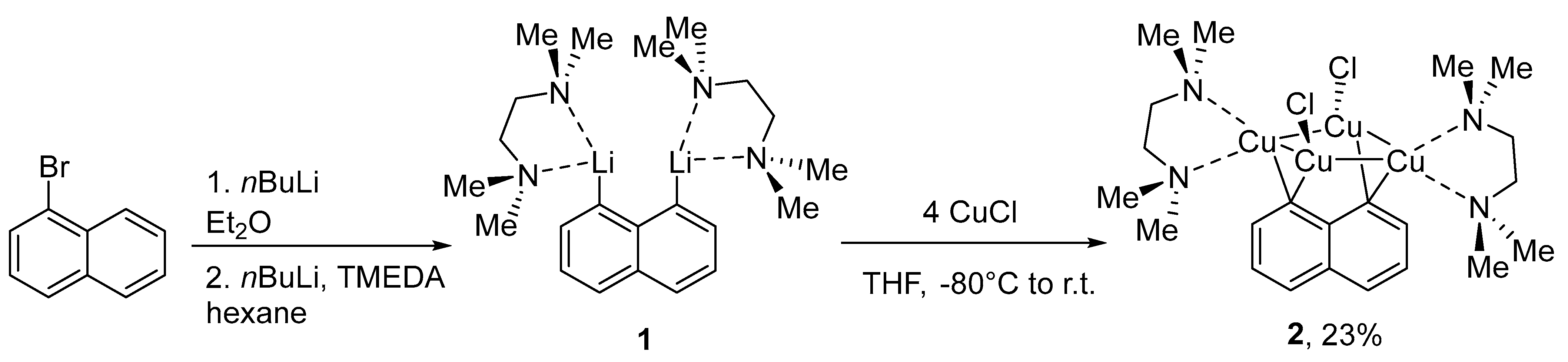
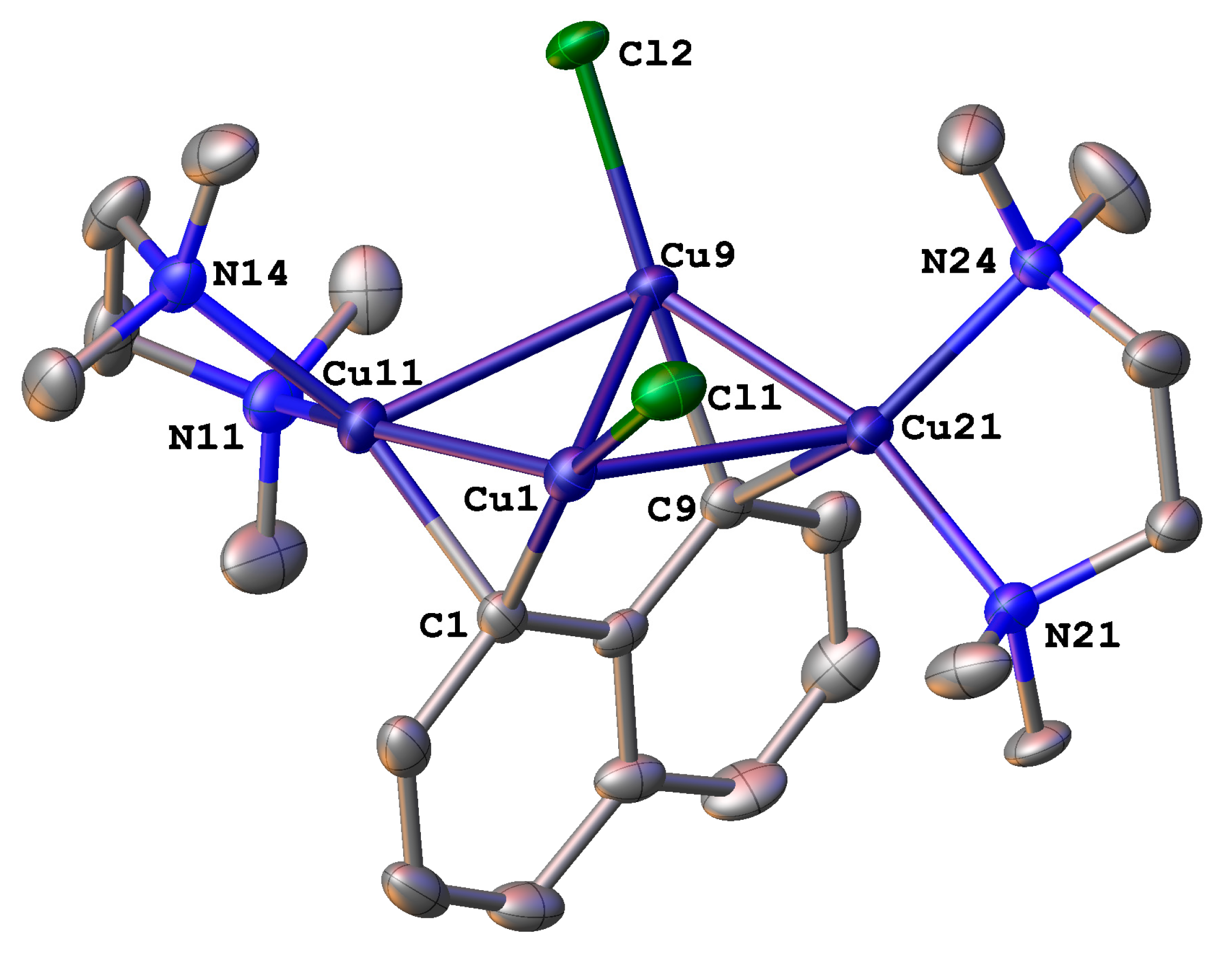
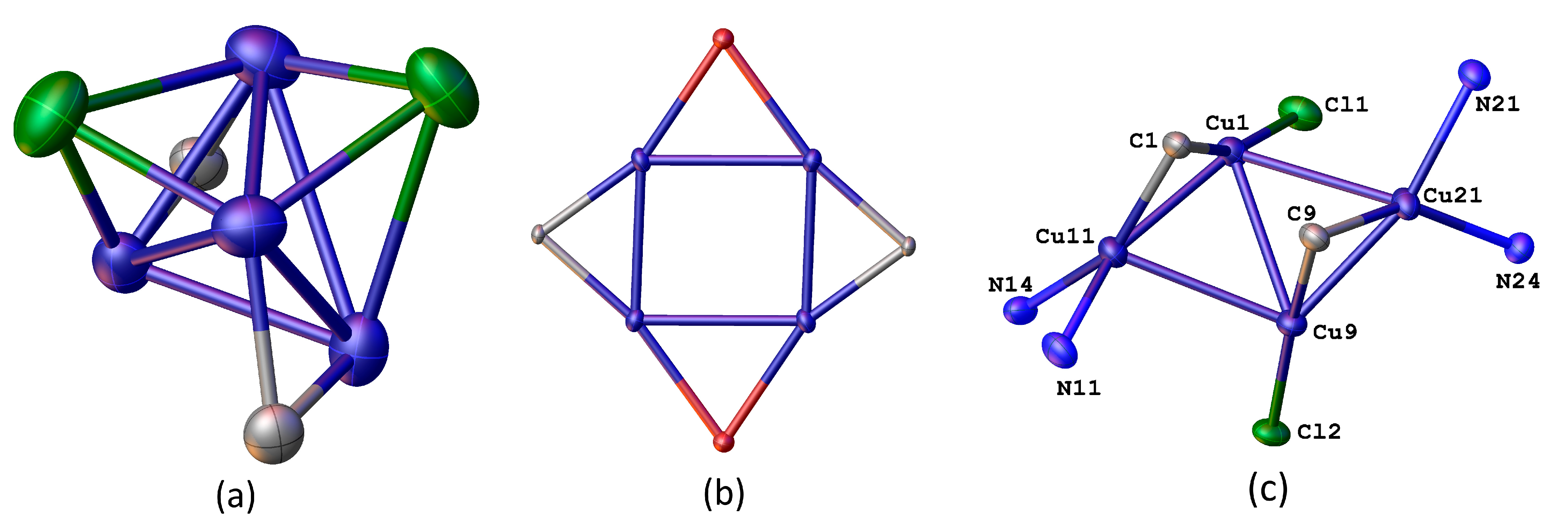
2. Results and Discussion
3. Materials and Methods
3.1. General Considerations
3.2. Synthesis of 1, NapLi2∙(TMEDA)2
3.3. Synthesis of 2, Nap(Cu4Cl2)(TMEDA)2
3.4. Crystallographic Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Krause, N.; Gerold, A. Regio- and Stereoselective Syntheses with Organocopper Reagents. Angew. Chem. Int. Ed. Engl. 1997, 36, 186–204. [Google Scholar] [CrossRef]
- Alexakis, A.; Benhaim, C. Enantioselective Copper-Catalysed Conjugate Addition. Eur. J. Org. Chem. 2002, 2002, 3221–3236. [Google Scholar] [CrossRef]
- Eriksson, H.; Håkansson, M.; Jagner, S. Pentamethylphenylcopper(I): A square-planar tetranuclear cluster. Inorganica Chim. Acta 1998, 277, 233–236. [Google Scholar] [CrossRef]
- Håkansson, M.; Eriksson, H.; Jagner, S. Tetranuclear homoleptic copper and silver aryls: 2,4,6-Triethylphenylcopper and 2,4,6-triethylphenylsilver. Inorganica Chim. Acta 2006, 359, 2519–2524. [Google Scholar] [CrossRef]
- Boeré, R.T.; Masuda, J.D.; Tran, P. Synthesis, crystal structure and DFT calculations on 2,6-diisopropylphenylcopper; its use in the preparation of dichloro-2,6-diisopropylphenylphosphine. J. Organomet. Chem. 2006, 691, 5585–5591. [Google Scholar] [CrossRef]
- Sundararaman, A.; Lalancette, R.A.; Zakharov, L.N.; Rheingold, A.L.; Jäkle, F. Structural Diversity of Pentafluorophenylcopper Complexes. First Evidence of π-Coordination of Unsupported Arenes to Organocopper Aggregates. Organometallics 2003, 22, 3526–3532. [Google Scholar] [CrossRef]
- Eriksson, H.; Håkansson, M. Mesitylcopper: Tetrameric and Pentameric. Organometallics 1997, 16, 4243–4244. [Google Scholar] [CrossRef]
- Håkansson, M.; Eriksson, H.; Åhman, A.B.; Jagner, S. Tetrameric thienylcopper and pentanuclear thienylcuprate. J. Organomet. Chem. 2000, 595, 102–108. [Google Scholar] [CrossRef]
- Guss, J.M.; Mason, R.; Søtofte, I.; van Koten, G.; Noltes, J.G. A tetranuclear cluster complex of copper(I) with bridging aryl ligands: The crystal structure of (4-methyl-2-cupriobenzyl)dimethylamine. J. Chem. Soc. Chem. Commun. 1972, 8, 446–447. [Google Scholar] [CrossRef]
- Wehman, E.; van Koten, G.; Knotter, M.; Spelten, H.; Heijdenrijk, D.; Mak, A.N.S.; Stam, C.H. 8-(Dimethylamino)naphthylcopper(I), a novel stable organocopper compound with unusual structural features. Its synthesis, crystal structure (X-ray), and reactivity. J. Organomet. Chem. 1987, 325, 293–309. [Google Scholar] [CrossRef]
- Nesmeyanov, A.N.; Struchkov, Y.T.; Sedova, N.N.; Andrianov, V.G.; Volgin, Y.V.; Sazonova, V.A. On the structure and properties of 2-copper-1-(dimethylaminomethyl)ferrocene. J. Organomet. Chem. 1977, 137, 217–221. [Google Scholar] [CrossRef]
- Li, B.; Zhang, C.; Yang, Y.; Zhu, H.; Roesky, H.W. Synthesis and Characterization of Heterobimetallic Al–O–Cu Complexes toward Models for Heterogeneous Catalysts on Metal Oxide Surfaces. Inorg. Chem. 2015, 54, 6641–6646. [Google Scholar] [CrossRef] [PubMed]
- Liebing, P.; Merzweiler, K. cyclo-Tetrakis([mu]-2,4,6-trimethylphenyl-[kappa]C1:[kappa]C1)bis(trimethylphosphane)-1[kappa]P,3[kappa]P-tetracopper(I). IUCrData 2021, 6, x210594. [Google Scholar] [CrossRef] [PubMed]
- Meyer, E.M.; Gambarotta, S.; Floriani, C.; Chiesi-Villa, A.; Guastini, C. Polynuclear aryl derivatives of Group 11 metals. Synthesis, solid state-solution structural relationship, and reactivity with phosphines. Organometallics 1989, 8, 1067–1079. [Google Scholar] [CrossRef]
- Furan, S.; Molkenthin, M.; Winkels, K.; Lork, E.; Mebs, S.; Hupf, E.; Beckmann, J. Tris(6-diphenylphosphinoacenaphth-5-yl)gallium: Z-Type Ligand and Transmetalation Reagent. Organometallics 2021, 40, 3785–3796. [Google Scholar] [CrossRef]
- Körte, L.A.; Schwabedissen, J.; Soffner, M.; Blomeyer, S.; Reuter, C.G.; Vishnevskiy, Y.V.; Neumann, B.; Stammler, H.-G.; Mitzel, N.W. Tris(perfluorotolyl)borane—A Boron Lewis Superacid. Angew. Chem. Int. Ed. 2017, 56, 8578–8582. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Huang, Z.; Wu, W.; Hong, D.; Zhu, S.; Wei, J.; Zhou, S.; Wang, S. Halide-bridged tetranuclear organocopper(i) clusters supported by indolyl-based NCN pincer ligands and their catalytic activities towards the hydrophosphination of alkenes. Dalton Trans. 2022, 51, 17795–17803. [Google Scholar] [CrossRef] [PubMed]
- Brandsma, L.; Verkruijsse, H.D. Preparative Polar Organometallic Chemistry; Springer: Berlin, Germany, 1987; Volume 1. [Google Scholar]
- Soolingen, J.v.; De Lang, R.J.; Besten, R.d.; Klusener, P.A.A.; Veldman, N.; Spek, A.L.; Brandsma, L. A Simple Procedure for the Preparation of 1,8-Bis(Diphenylphosphino)Naphthalene. Synth. Commun. 1995, 25, 1741–1744. [Google Scholar] [CrossRef]
- Costa, T.; Schmidbaur, H. 1,8-Naphthalindiylbis(dimethylphosphan): Konsequenzen sterischer Hinderung für Methylierung und Borylierung. Chem. Berichte 1982, 115, 1374–1378. [Google Scholar] [CrossRef]
- Karaçar, A.; Thönnessen, H.; Jones, P.G.; Bartsch, R.; Schmutzler, R. 1,8-Bis(phosphino)naphthalenes: Synthesis and molecular structures. Heteroat. Chem. 1997, 8, 539–550. [Google Scholar] [CrossRef]
- Jackson, R.D.; James, S.; Orpen, A.G.; Pringle, P.G. 1,8-Bis(diphenylphosphino)naphthalene: A rigid chelating, diphosphine analogue of proton sponge. J. Organomet. Chem. 1993, 458, C3–C4. [Google Scholar] [CrossRef]
- Karaçar, A.; Freytag, M.; Thönnessen, H.; Jones, P.G.; Bartsch, R.; Schmutzler, R. Preparation and structures of 1-dimethylamino-2-bis(dimethylamino)- and 1-chloro-2-bis(diethylamino)-1-phospha-2-phosphonium acenaphthene: The first examples of the 1,2-dihydro-1,2-diphospha-acenaphthene ring system. J. Organomet. Chem. 2002, 643–644, 68–80. [Google Scholar] [CrossRef]
- Mizuta, T.; Nakazono, T.; Miyoshi, K. Naphtho[1,8-b,c]phosphete and 1,2-Diphosphaacenaphthene from the Reaction of 1,8-Dilithionaphthalene with RPCl2. Angew. Chem. Int. Ed. 2002, 41, 3897–3898. [Google Scholar] [CrossRef]
- Mizuta, T.; Iwakuni, Y.; Nakazono, T.; Kubo, K.; Miyoshi, K. Preparation and reaction of phosphorus peri-bridged naphthalenes and their adducts with Lewis acids. J. Organomet. Chem. 2007, 692, 184–193. [Google Scholar] [CrossRef]
- Ito, M.; Hashizume, D.; Fukunaga, T.; Matsuo, T.; Tamao, K. Isolated Monomeric and Dimeric Mixed Diorganocuprates Based on the Size-Controllable Bulky “Rind” Ligands. J. Am. Chem. Soc. 2009, 131, 18024–18025. [Google Scholar] [CrossRef]
- CrystalClear-SM Expert, v2.0; Rigaku Americas: The Woodlands, TX, USA; Rigaku Corporation: Tokyo, Japan, 2011.
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; De Caro, L.; Giacovazzo, C.; Polidori, G.; Spagna, R. SIR2004: An improved tool for crystal structure determination and refinement. J. Appl. Crystallogr. 2005, 38, 381–388. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallographica. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- CrystalStructure 4.3.0; Rigaku Americas: The Woodlands, TX, USA; Rigaku Corporation: Tokyo, Japan, 2018.
- Ray, M.J.; Saviantoni, M.L.; Slawin, A.M.Z.; Cordes, D.B.; Kilian, P. Dichloro-μ2,μ2-naphthalene-1,8-diyl-bis(N,N,N’,N’-tetramethylethylenediamino)tetracopper(I) Dataset; University of St Andrews Research Portal: St Andrews, UK, 2024. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ray, M.J.; Saviantoni, M.L.; Slawin, A.M.Z.; Cordes, D.B.; Kilian, P. Dichloro-μ2,μ2-naphthalene-1,8-diyl-bis(N,N,N′,N′-tetramethylethylenediamino)tetracopper(I). Molbank 2024, 2024, M1832. https://doi.org/10.3390/M1832
Ray MJ, Saviantoni ML, Slawin AMZ, Cordes DB, Kilian P. Dichloro-μ2,μ2-naphthalene-1,8-diyl-bis(N,N,N′,N′-tetramethylethylenediamino)tetracopper(I). Molbank. 2024; 2024(2):M1832. https://doi.org/10.3390/M1832
Chicago/Turabian StyleRay, Matthew J., Maria Laura Saviantoni, Alexandra M. Z. Slawin, David B. Cordes, and Petr Kilian. 2024. "Dichloro-μ2,μ2-naphthalene-1,8-diyl-bis(N,N,N′,N′-tetramethylethylenediamino)tetracopper(I)" Molbank 2024, no. 2: M1832. https://doi.org/10.3390/M1832
APA StyleRay, M. J., Saviantoni, M. L., Slawin, A. M. Z., Cordes, D. B., & Kilian, P. (2024). Dichloro-μ2,μ2-naphthalene-1,8-diyl-bis(N,N,N′,N′-tetramethylethylenediamino)tetracopper(I). Molbank, 2024(2), M1832. https://doi.org/10.3390/M1832







