Synthesis of 2-[(3,4,5-Triphenyl)phenyl]acetic Acid and Derivatives
Abstract
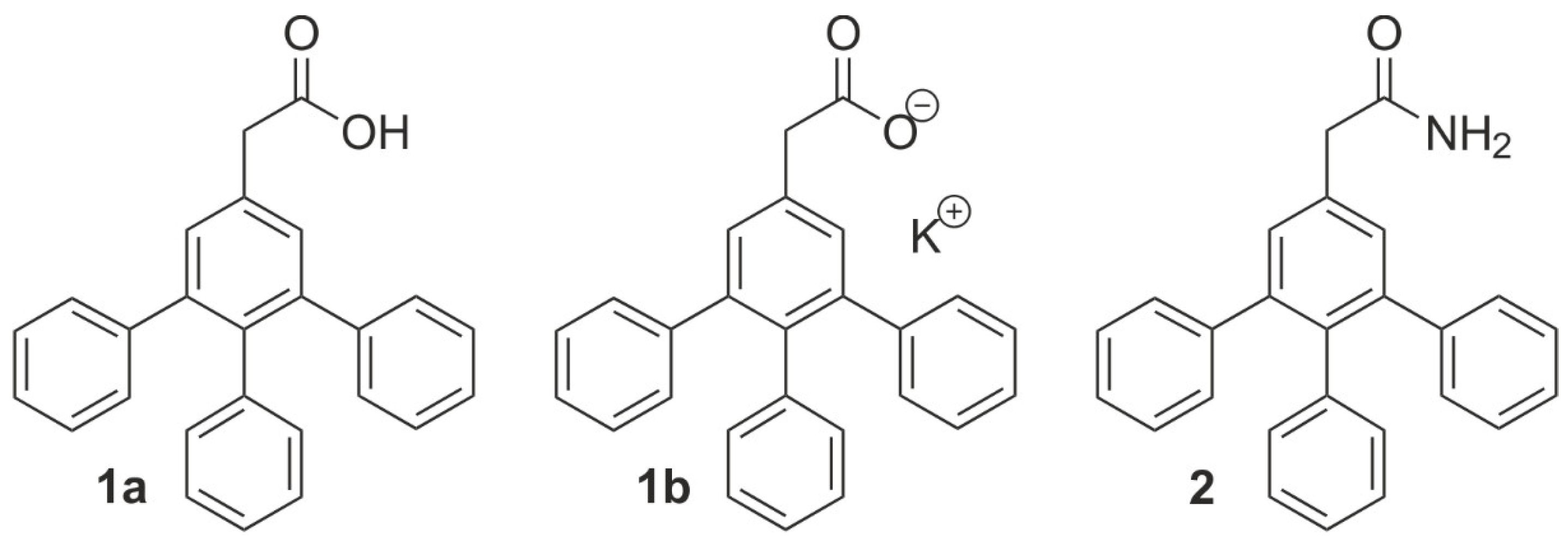
:1. Introduction
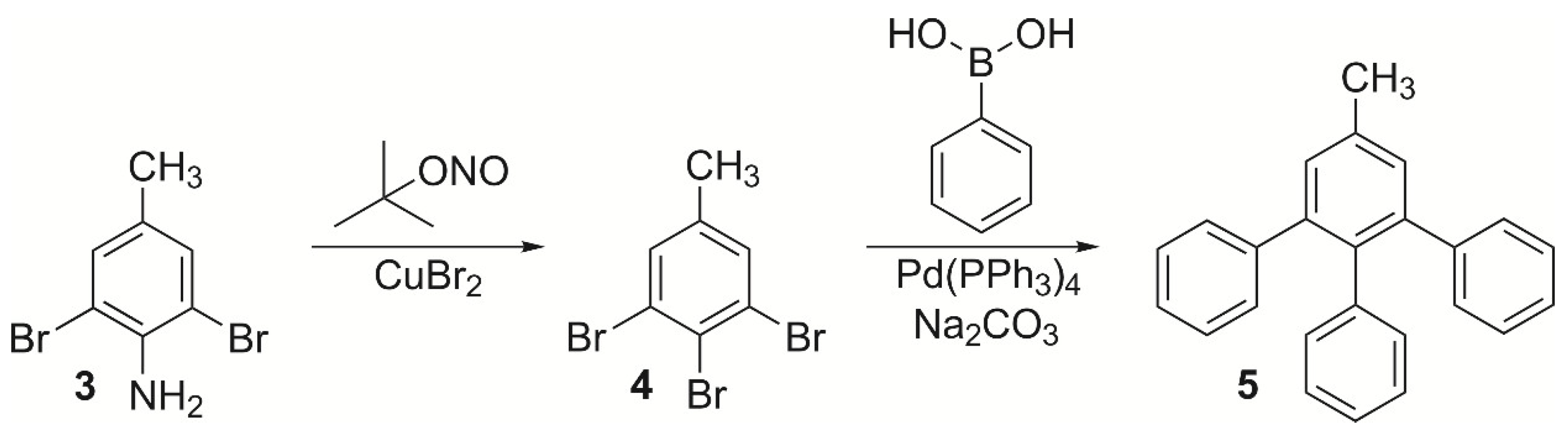
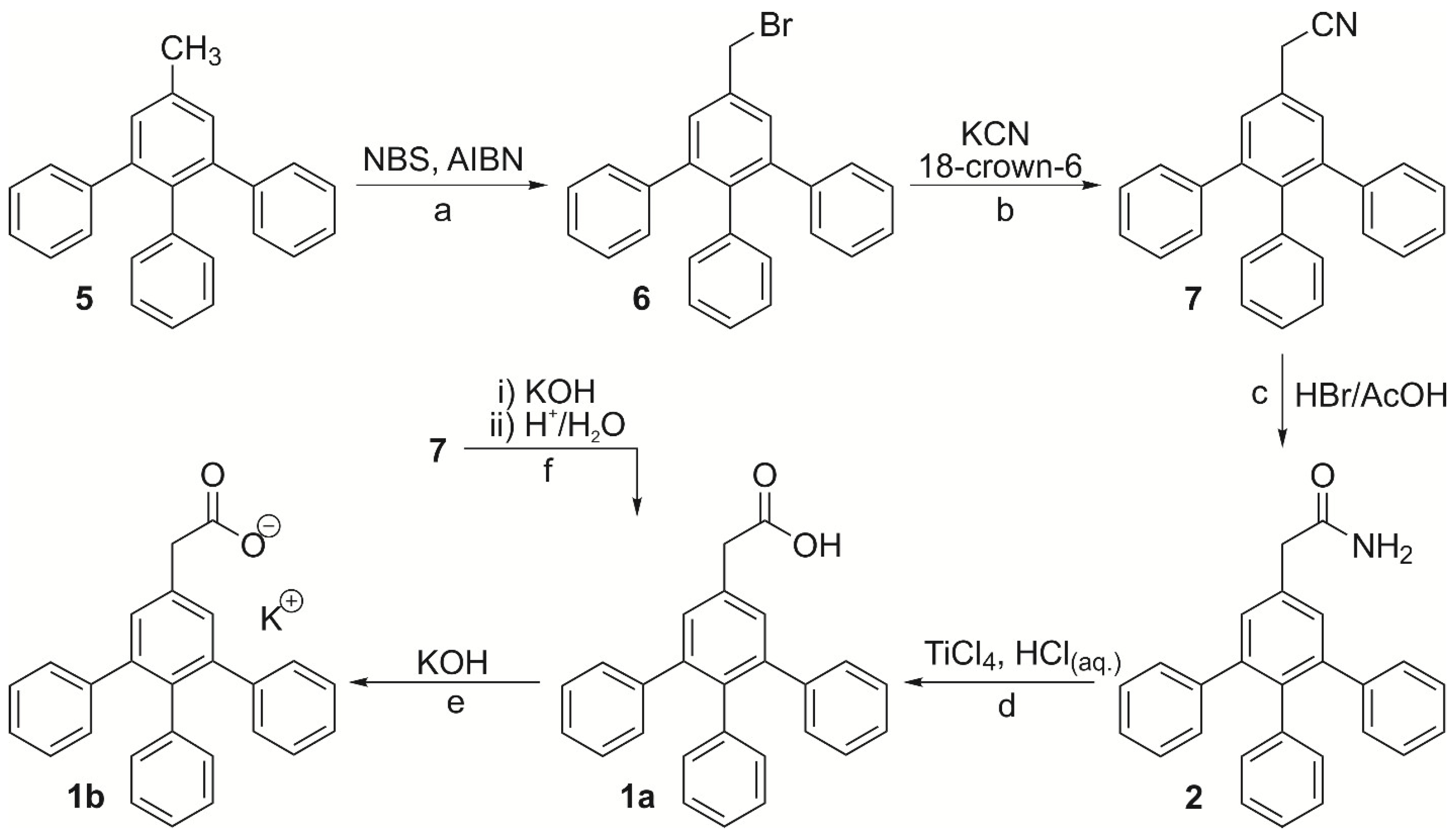
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Preparation of Compounds 1a, 1b and 2–7
4.1.1. 3,4,5-Triphenyltoluene (5)
4.1.2. 3,4,5-Triphenylbenzyl Bromide (6)
4.1.3. 2-[(3,4,5-Triphenyl)phenyl]acetonitrile (7)
4.1.4. 2-[(3,4,5-Triphenyl)phenyl]acetamide (2)
4.1.5. 2-[(3,4,5-Triphenyl)phenyl]acetic Acid (1a)
4.1.6. Potassium 2-[(3,4,5-Triphenyl)phenyl]acetate (1b)
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cook, S.D. An Historical Review of Phenylacetic Acid. Plant Cell Physiol. 2019, 60, 243–254. [Google Scholar] [CrossRef]
- Perez, V.C.; Zhao, H.; Lin, M.; Kim, J. Occurrence, Function, and Biosynthesis of the Natural Auxin Phenylacetic Acid (PAA) in Plants. Plants 2023, 12, 266. [Google Scholar] [CrossRef] [PubMed]
- Jiao, M.; He, W.; Ouyang, Z.; Shi, Q.; Wen, Y. Progress in structural and functional study of the bacterial phenylacetic acid catabolic pathway, its role in pathogenicity and antibiotic resistance. Front. Microbiol. 2022, 13, 964019–964036. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Q.; Pan, H.; Li, X.; Guo, D. Metabolic engineering of Escherichia coli to high efficient synthesis phenylacetic acid from phenylalanine. AMB Express 2017, 7, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Cáceres-Jiménez, S.; Rodríguez-Solana, R.; Dobani, S.; Pourshahidi, K.; Gill, C.; Moreno-Rojas, J.M.; Almutairi, T.M.; Crozier, A.; Pereira-Caro, G. UHPLC-HRMS Spectrometric Analysis: Method Validation and Plasma and Urinary Metabolite Identification after Mango Pulp Intake. J. Agric. Food Chem. 2023, 71, 11520–11533. [Google Scholar] [CrossRef]
- Eisenhofer, G.; Kopin, I.J.; Goldstein, D.S. Catecholamine metabolism: A contemporary view with implications for physiology and medicine. Pharmacol. Rev. 2004, 56, 331–349. [Google Scholar] [CrossRef]
- Griffin, L.E.; Kohrt, S.E.; Rathore, A.; Kay, C.D.; Grabowska, M.M.; Neilson, A.P. Microbial Metabolites of Flavanols in Urine are Associated with Enhanced Anti-Proliferative Activity in Bladder Cancer Cells In Vitro. Nutr. Cancer 2022, 74, 194–210. [Google Scholar] [CrossRef]
- Olthof, M.R.; Hollman, P.C.H.; Buijsman, M.N.C.P.; van Amelsvoort, J.M.M.; Katan, M.B. Chlorogenic acid, quercetin-3-rutinoside and black tea phenols are extensively metabolized in humans. J. Nutr. 2003, 133, 1806–1814. [Google Scholar] [CrossRef]
- Gao, K.; Xu, A.; Krul, C.; Venema, K.; Liu, Y.; Niu, Y.; Lu, J.; Bensoussan, L.; Seeram, N.P.; Heber, D.; et al. Of the Major Phenolic Acids Formed during Human Microbial Fermentation of Tea, Citrus, and Soy Flavonoid Supplements, Only 3,4-Dihydroxyphenylacetic Acid Has Antiproliferative Activity. J. Nutr. 2006, 136, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Mazik, M. Promising Therapeutic Approach for SARS-CoV-2 Infections by Using a Rutin-Based Combination Therapy. ChemMedChem 2022, 17, e202200157. [Google Scholar] [CrossRef]
- Agrawal, P.K.; Agrawal, C.; Blunden, G. Rutin: A Potential Antiviral for Repurposing as a SARS-CoV-2 Main Protease (Mpro) Inhibitor. Nat. Prod. Commun. 2021, 16, 1–12. [Google Scholar] [CrossRef]
- Enns, G.M.; Berry, S.A.; Berry, G.T.; Rhead, W.J.; Brusilow, S.W.; Hamosh, A. Survival after treatment with phenylacetate and benzoate for urea-cycle disorders. N. Engl. J. Med. 2007, 356, 2282–2292. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, P.; Thibault, A.; Liu, L.; Samid, D. Lipid metabolism as a target for brain cancer therapy: Synergistic activity of lovastatin and sodium phenylacetate against human glioma cells. J. Neurochem. 1996, 66, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Aliabadi, A.; Andisheh, S.; Tayarani-Najaran, Z.; Tayarani-Najaran, M. 2-(4-Fluorophenyl)-N-phenylacetamide Derivatives as Anticancer Agents: Synthesis and In-vitro Cytotoxicity Evaluation. Iran. J. Pharm. Res. 2013, 12, e125688. [Google Scholar]
- Lade, D.M.; Nicoletti, R.; Mersch, J.; Agazie, Y.M. Design and synthesis of improved active-site SHP2 inhibitors with anti-breast cancer cell effects. Eur. J. Med. Chem. 2023, 247, 115017–115030. [Google Scholar] [CrossRef] [PubMed]
- Greig, N.H.; Daly, E.M.; Sweeney, D.J.; Rapoport, S.I. Pharmacokinetics of chlorambucil-tertiary butyl ester, a lipophilic chlorambucil derivative that achieves and maintains high concentrations in brain. Cancer Chemother. Pharmacol. 1990, 25, 320–325. [Google Scholar] [CrossRef] [PubMed]
- El-Araby, M.E.; Omar, A.M.; Khayat, M.T.; Shah, D.; Safo, M.K.; Malebari, A.M.; Ahmed, F. Amide Group-Containing Compounds and Use for Cancer Treatment. U.S. Patent 10844007B1, 24 November 2020. [Google Scholar]
- Wilson, F.; Reid, A.; Reader, V.; Harrison, R.J.; Sunose, M.; Hernadez, P.R.; Major, J.; Boussard, C.; Smelt, K.; Taylor, J.; et al. Terphenyl Derivatives for Treatment of Alzheimer’s Disease. KR Patent 101494906B1, 24 February 2015. [Google Scholar]
- Chandrasoma, N.; Brown, N.; Brassfield, A.; Nerurkar, A.; Suarez, S.; Buszek, K.R. Total synthesis of (±)-cis-trikentrin B via intermolecular 6,7-indole aryne cycloaddition and Stille cross-coupling. Tetrahedron Lett. 2013, 54, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Feng, J.; Liu, S.; Guo, W.; Wang, Z.; Bian, K.; Sun, J. Polycyclic Aromatic Compound and Organic Electroluminescent Element. CN Patent 113461718A, 1 October 2021. [Google Scholar]
- Mao, Y.; Chen, W.; Li, C.; Miao, L.; Lin, Y.; Ling, F.; Chen, Z.; Yao, J. Synthesis of 3,4,5-trisubstituted phenols via Rh(III)-catalyzed alkenyl C–H activation assisted by phosphonium cations. Chem. Commun. 2023, 59, 3775–3778. [Google Scholar] [CrossRef] [PubMed]
- Dötz, F.; Brand, J.D.; Ito, S.; Gherghel, L.; Müllen, K. Synthesis of Large Polycyclic Aromatic Hydrocarbons: Variation of Size and Periphery. J. Am. Chem. Soc. 2000, 122, 7707–7717. [Google Scholar] [CrossRef]
- Giroux, A.; Nadeau, C.; Han, Y. Synthesis of phenylacetic acids under rhodium-catalyzed carbonylation conditions. Tetrahedron Lett. 2000, 41, 7601–7604. [Google Scholar] [CrossRef]
- Yamabe, H.; Okuyama, M.; Nakao, A.; Ooizumi, M.; Saito, K.-I. Novel Cyclic Amide Derivatives. U.S. Patent 2003212094A1, 23 January 2003. [Google Scholar]
- Nimgirawath, S.; Lorpitthaya, R.; Wanbanjob, A.; Taechowisan, T.; Shen, Y.-M. Total Synthesis and the Biological Activities of (±)-Norannuradhapurine. Molecules 2008, 14, 89–101. [Google Scholar] [CrossRef] [PubMed]
- McNaughton-Smith, G.A.; Burns, J.F.; Stocker, J.W.; Rigdon, G.C.; Creech, C.; Arrington, S.; Shelton, T.; de Franceschi, L. Novel inhibitors of the Gardos channel for the treatment of sickle cell disease. J. Med. Chem. 2008, 51, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Fisher, L.E.; Caroon, J.M.; Stabler, S.R.; Lundberg, S.; Zaidi, S.; Sorensen, C.M.; Sparacino, M.L.; Muchowski, J.M. Mild hydrolysis or alcoholysis of amides. Ti(IV) catalyzed conversion of primary carboxamides to carboxylic acids or esters. Can. J. Chem. 1994, 72, 142–145. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazik, M.; Seidel, P. Synthesis of 2-[(3,4,5-Triphenyl)phenyl]acetic Acid and Derivatives. Molbank 2024, 2024, M1837. https://doi.org/10.3390/M1837
Mazik M, Seidel P. Synthesis of 2-[(3,4,5-Triphenyl)phenyl]acetic Acid and Derivatives. Molbank. 2024; 2024(2):M1837. https://doi.org/10.3390/M1837
Chicago/Turabian StyleMazik, Monika, and Pierre Seidel. 2024. "Synthesis of 2-[(3,4,5-Triphenyl)phenyl]acetic Acid and Derivatives" Molbank 2024, no. 2: M1837. https://doi.org/10.3390/M1837





