Structural Elucidation of a New Puzzling Compound Emerged from Doebner Quinoline Synthesis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Spectral Analysis
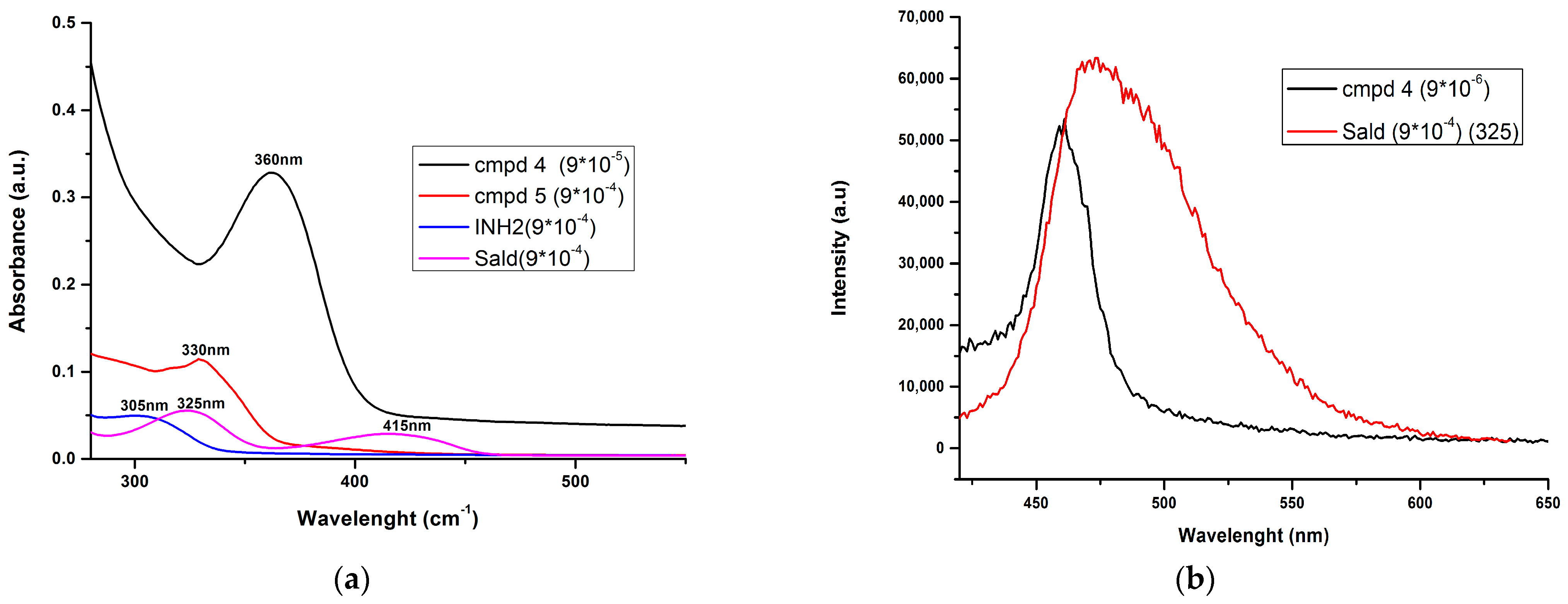
2.3. Photophysical Investigations
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, S.; Bawa, S.; Gupta, H. Biological activities of quinoline derivatives. Mini-Rev. Med. Chem. 2009, 9, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, J.; Ortmann, R.; Jomaa, H.; Schlitzer, M. New antimalarial drugs. Angew. Chem. Int. Ed. 2003, 42, 5274–5293. [Google Scholar] [CrossRef] [PubMed]
- Clemence, F.; Martret, O.L.; Delevallee, F.; Benzoni, J.; Jouanen, A.; Jouquey, S.; Mouren, M.; Deraedt, R. 4-Hydroxy-3-quinolinecarboxamides with antiarthritic and analgesic activities. J. Med. Chem. 1988, 31, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, W.; Carlson, R.P.; Crossley, R.; Datko, L.J.; Dietrich, S.; Heatherington, K.; Marshall, L.A.; Meade, P.J.; Opalko, A.; Shepherd, R.G. Synthesis and antiinflammatory activity of certain 5, 6, 7, 8-tetrahydroquinolines and related compounds. J. Med. Chem. 1995, 38, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Kohno, Y.; Awano, K.; Miyashita, M.; Ishizaki, T.; Kuriyama, K.; Sakoe, Y.; Kudoh, S.; Saito, K.; Kojima, E. Synthesis and antirheumatic activity of novel tetrahydro-6-quinolineacetic acid derivatives. Bioorg. Med. Chem. Lett. 1997, 7, 1519–1524. [Google Scholar] [CrossRef]
- Guo, L.J.; Wei, C.X.; Jia, J.H.; Zhao, L.M.; Quan, Z.S. Design and synthesis of 5-alkoxy-[1,2,4]triazolo[4,3-a]quinoline derivatives with anticonvulsant activity. Eur. J. Med. Chem. 2009, 44, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Shabana, K.; Shabana; Mazumder, A.; Singh, H.; Kumar, R.; Tyagi, S.; Datt, V.; Sharma, A.S.; Yar, M.S.; Ahsan, M.J.; et al. Synthesis, characterization, in silico and in vivo evaluation of benzimidazole-bearing quinoline schiff bases as new anticonvulsant agents. ChemistrySelect 2023, 8, e202300209. [Google Scholar] [CrossRef]
- Ferlin, M.G.; Chiarelotto, G.; Antonucci, F.; Caparrotta, L.; Froldi, G. Mannich bases of 3H-pyrrolo[3,2-f]quinoline having vasorelaxing activity. Eur. J. Med. Chem. 2002, 37, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Sircar, I.; Haleen, S.J.; Burke, S.E.; Barth, H. Synthesis and biological activity of 4-(diphenylmethyl)-alpha-[(4-quinolinyloxy)methyl]-1-piperazineethanol and related compounds. J. Med. Chem. 1992, 35, 4442–4449. [Google Scholar] [CrossRef]
- Chaaban, I.; Hafez, H.; AlZaim, I.; Tannous, C.; Ragab, H.; Hazzaa, A.; Ketat, S.; Ghoneim, A.; Katary, M.; Abd-Alhaseeb, M.M.; et al. Transforming iodoquinol into broad spectrum anti-tumor leads: Repurposing to modulate redox homeostasis. Bioorg. Chem. 2021, 113, 105035. [Google Scholar] [CrossRef]
- Kemnitzer, W.; Kuemmerle, J.; Jiang, S.; Zhang, H.Z.; Sirisoma, N.; Kasibhatla, S.; Crogan-Grundy, C.; Tseng, B.; Drewe, J.; Cai, X.Z. Discovery of 1-benzoyl-3-cyanopyrrolo[1,2-a]quinolines as a new series of apoptosis inducers using a cell- and caspase-based high-throughput screening assay. Part 1: Structure–activity relationships of the 1- and 3-positions. Bioorg. Med. Chem. Lett. 2008, 18, 6259–6264. [Google Scholar] [CrossRef]
- Köprülü, T.K.; Ökten, S.; Tekin, S.; Çakmak, O. Biological evaluation of some quinoline derivatives with different functional groups as anticancer agents. J. Biochem. Mol. Toxicol. 2019, 33, e22260. [Google Scholar] [CrossRef]
- Bokosi, F.R.B.; Beteck, R.M.; Jordaan, A.; Seldon, R.; Warner, D.F.; Tshiwawa, T.; Lobb, K.; Khanye, S.D. Arylquinolinecarboxamides: Synthesis, in vitro and in silico studies against Mycobacterium tuberculosis. J. Heterocycl. Chem. 2021, 58, 2140–2151. [Google Scholar] [CrossRef]
- Santoso, K.T.; Menorcad, A.; Cheungd, C.Y.; Cook, G.M.; Stocker, B.L.; Timmer, M.S.M. The synthesis and evaluation of quinolinequinones as anti-mycobacterial agents. Bioorg. Med. Chem. 2019, 27, 3532–3545. [Google Scholar] [CrossRef]
- Ghareeb, E.A.; Mahmoud, N.F.H.; El-Bordany, E.A.; El-Helw, E.A.E. Synthesis, DFT, and eco-friendly insecticidal activity of some N-heterocycles derived from 4-((2-oxo-1,2-dihydroquinolin-3-yl)methylene)-2-phenyloxazol-5(4H)-one. Bioorg. Chem. 2021, 112, 104945. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Ma, K.-Y.; Du, S.-S.; Zhang, Z.-J.; Wu, T.-L.; Sun, Y.; Liu, Y.-Q.; Yin, X.-D.; Zhou, R.; Yan, Y.-F.; et al. Antifungal exploration of quinoline derivatives against phytopathogenic fungi inspired by quinine alkaloids. J. Agric. Food Chem. 2021, 69, 12156–12170. [Google Scholar] [CrossRef]
- Li, G.; Zhu, D.; Xue, L.; Jiang, H. Quinoline-based fluorescent probe for ratiometric detection of lysosomal pH. Org. Lett. 2013, 15, 5020–5023. [Google Scholar] [CrossRef]
- Van den Berg, O.; Jager, W.F.; Picken, S.J. 7-Dialkylamino-1-alkylquinolinium salts: Highly versatile and stable fluorescent probes. J. Org. Chem. 2006, 71, 2666–2676. [Google Scholar] [CrossRef]
- Liu, X.; Su, Y.; Tian, H.; Yang, L.; Zhang, H.; Song, X.; Foley, J.W. Ratiometric fluorescent probe for lysosomal pH measurement and imaging in living cells using single-wavelength excitation. Anal. Chem. 2017, 89, 7038–7045. [Google Scholar] [CrossRef]
- El-Ghamaz, N.A.; Diab, M.A.; El-Bindary, A.A.; El-Sonbati, A.Z.; Nozha, S.G. Thermal, dielectric characteristics and conduction mechanism of azodyes derived from quinoline and their copper complexes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 143, 200–212. [Google Scholar] [CrossRef]
- Jiang, B.; Ning, X.; Gong, S.; Jiang, N.; Zhong, C.; Lu, Z.-H.; Yang, C. Highly efficient red iridium(III) complexes cyclometalated by 4-phenylthieno[3,2-c]quinoline ligands for phosphorescent OLEDs with external quantum efficiencies over 20%. J. Mater. Chem. C 2017, 5, 10220–10224. [Google Scholar] [CrossRef]
- Sych, G.; Volyniuk, D.; Bezvikonnyi, O.; Lytvyn, R.; Grazulevicius, J.V. Dual interface exciplex emission of quinoline and carbazole derivatives for simplified nondoped white OLEDs. J. Phys. Chem. C 2019, 123, 2386–2397. [Google Scholar] [CrossRef]
- Aoki, S.; Sakurama, K.; Matsuo, N.; Yamada, Y.; Takasawa, R.; Tanuma, S.; Shiro, M.; Takeda, K.; Kimura, E. A new fluorescent probe for Zinc(II): An 8-hydroxy-5-N,N-dimethylaminosulfonylquinoline-pendant 1,4,7,10-Tetraazacyclododecane. Chem. Eur. J. 2006, 12, 9066–9080. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Dong, Y.; Zhang, B.; Liu, F.; Tan, C.; Tan, Y.; Jiang, Y. An efficient quinoline-based fluorescence sensor for zinc(II) and its application in live-cell imaging. Sens. Actuators B-Chem. 2016, 234, 616–624. [Google Scholar] [CrossRef]
- Kouznetsov, V.V.; Mendez, L.Y.V.; Gomez, C.M.M. Recent progress in the synthesis of quinolines. Curr. Org. Chem. 2005, 9, 141–161. [Google Scholar] [CrossRef]
- Prajapati, S.M.; Patel, K.D.; Vekariya, R.H.; Panchal, S.N.; Pate, H.D. Recent advances in the synthesis of quinolines: A review. RSC Adv. 2014, 4, 24463–24476. [Google Scholar] [CrossRef]
- Weyesa, A.; Mulugeta, E. Recent advances in the synthesis of biologically and pharmaceutically active quinoline and its analogues: A review. RSC Adv. 2020, 10, 20784–20793. [Google Scholar] [CrossRef]
- Yamashkin, S.A.; Oreshkina, E.A. Traditional and modern approaches to the synthesis of quinoline systems by the Skraup and Doebner–Miller methods. Chem. Heterocycl. Compd. 2006, 42, 701–718. [Google Scholar] [CrossRef]
- Al Matarneh, C.M.; Rosca, I.; Apostu, M.O.; Danac, R. Synthesis, structure, characterization and antibacterial evaluation of new [1,10]phenanthroline derivatives. J. Serb. Chem. Soc. 2021, 86, 901–915. [Google Scholar] [CrossRef]
- Al Matarneh, C.M.; Ciobanu, C.; Mangalagiu, V.; Zbancioc, G.; Danac, R. Microwave assisted synthesis of six member ring azaheterocycles and their antimycobacterial and anticancer evaluation. Rev. Chim. 2020, 71, 287–293. [Google Scholar] [CrossRef]
- Al-Matarneh, C.M.; Sardaru, M.C.; Apostu, M.O.; Rosca, I.; Ciobanu, C.I.; Mangalagiu, I.I.; Danac, R. Synthesis and antibacterial evaluation of new pyrrolo [3′,4′:3,4]pyrrolo[1,2-a]Quinoline and pyrrolo[3′,4′:3,4]pyrrolo[2,1-a]Isoquinoline derivatives. Stud. Univ. Babes-Bolyai Chem. 2019, 64, 67–80. [Google Scholar] [CrossRef]
- Al Matarneh, C.M.; Apostu, M.O.; Mangalagiu, I.I.; Danac, R. Reactions of ethyl cyanoformate with cycloimmonium salts: A direct pathway to fused or substituted azaheterocycles. Tetrahedron 2016, 72, 4230–4238. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Ba, Y.; Xu, Z. 1,2,4-Triazole-quinoline/quinolone hybrids as potential anti-bacterial agents. Eur. J. Med. Chem. 2019, 174, 1–8. [Google Scholar] [CrossRef]
- Mantu, D.; Antoci, V.; Nicolescu, A.; Delenu, C.; Vasilache, V.; Mangalagiu, I.I. Synthesis, stereochemical studies and antimycobacterial activity of new acetyl- hydrazine pyridazinones. Curr. Org. Synth. 2017, 14, 112–119. [Google Scholar] [CrossRef]
- Popovici, L.; Amarandi, R.M.; Mangalagiu, I.I.; Mangalagiu, V.; Danac, R. Synthesis, molecular modelling and anticancer evaluation of new pyrrolo[1,2-b]pyridazine and pyrrolo[2,1-a]phthalazine derivatives. J. Enz. Inhib. Med. Chem. 2019, 34, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Al-Matarneh, C.M.; Nicolescu, A.; Marinaş, I.C.; Găboreanu, M.D.; Shova, S.; Dascălu, A.; Silion, M.; Pinteală, M. New library of iodo-quinoline derivatives obtained by an alternative synthetic pathway and their antimicrobial activity. Molecules 2024, 29, 772. [Google Scholar] [CrossRef] [PubMed]
- Marek, R.; Lykab, A. 15N NMR spectroscopy in structural analysis. Curr. Org. Chem. 2002, 6, 35–66. [Google Scholar] [CrossRef]
- Georgescu, E.; Nicolescu, A.; Georgescu, F.; Shova, S.; Teodorescu, F.; Macsim, A.M.; Deleanu, C. Novel one-pot multicomponent strategy for the synthesis of pyrrolo[1,2-a] benzimidazole and pyrrolo[1,2-a] quinoxaline derivatives. Synthesis 2015, 47, 1643–1655. [Google Scholar] [CrossRef]
- Airinei, A.; Tigoianu, R.; Danac, R.; Al Matarneh, C.M.; Isac, D.L. Steady state and time resolved fluorescence studies of new indolizine derivatives with phenanthroline skeleton. J. Lumin. 2018, 199, 6–12. [Google Scholar] [CrossRef]
- Doroftei, C.; Leontie, L.; Danac, R.; Al Matarneh, C.M.; Carlescu, A. Exploring pyrrolo-phenanthrolines as semiconductors for potential implementation in organic electronics. Materials 2023, 16, 3366. [Google Scholar] [CrossRef]
- Coroaba, A.; Al-Matarneh, M.C.; Vasiliu, T.; Ibanescu, S.-A.; Zonda, R.; Esanu, I.; Isac, D.-L.; Pinteala, M. Revealing the supramolecular interactions of the bis(azopyrenyl) dibenzo-18-crown-6-ether system. J. Mol. Liq. 2023, 374, 121298. [Google Scholar] [CrossRef]
- Coroaba, A.; Isac, D.L.; Al-Matarneh, M.C.; Vasiliu, T.; Ibanescu, S.A.; Zonda, R.; Ardeleanu, R.; Neamtu, A.; Timpu, D.; Nicolescu, A.; et al. Probing the supramolecular features: Via π-π interaction of a di-iminopyrene-di-benzo-18-crown-6-ether compound: Experimental and theoretical study. RSC Adv. 2020, 10, 38304–38315. [Google Scholar] [CrossRef] [PubMed]


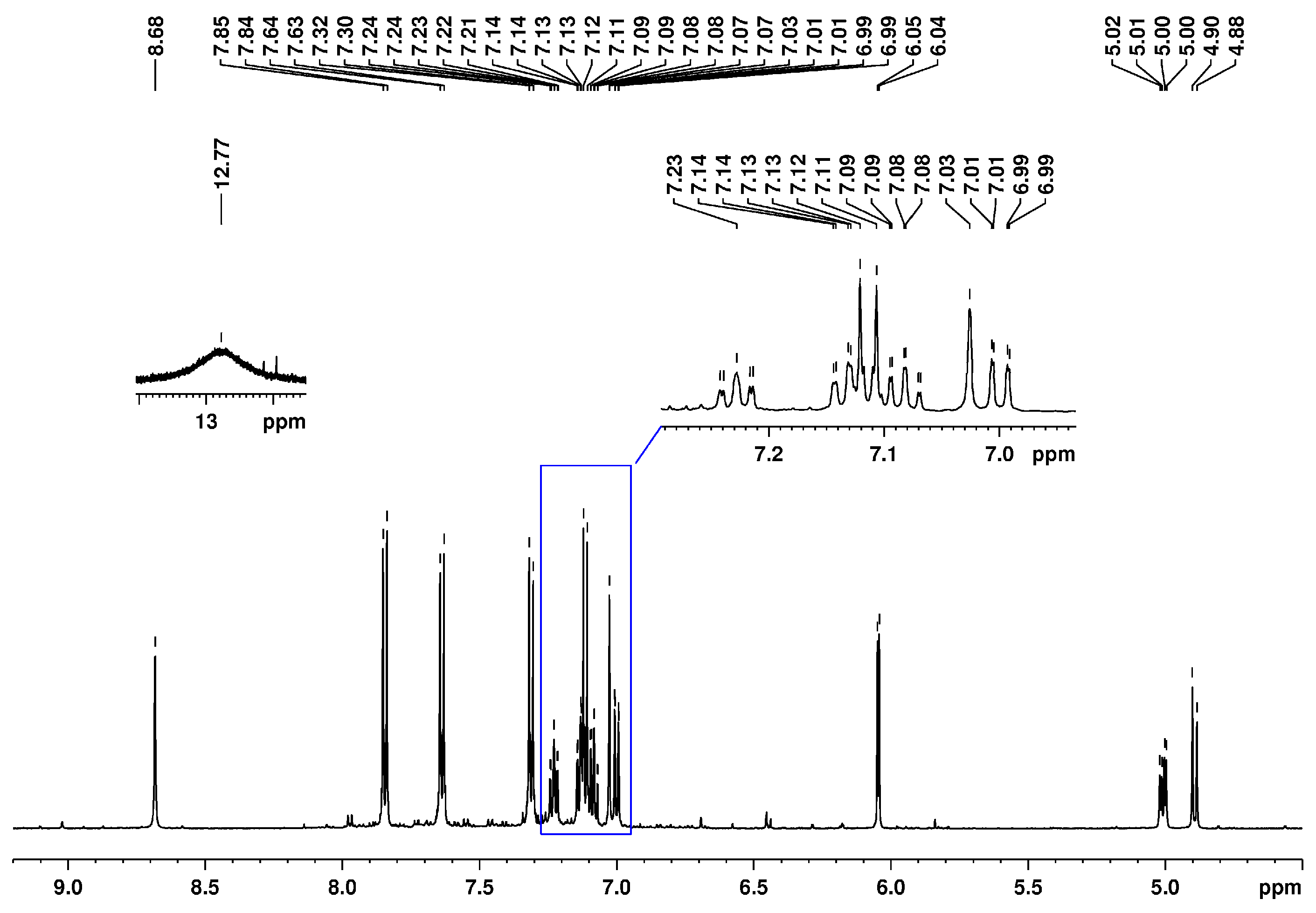
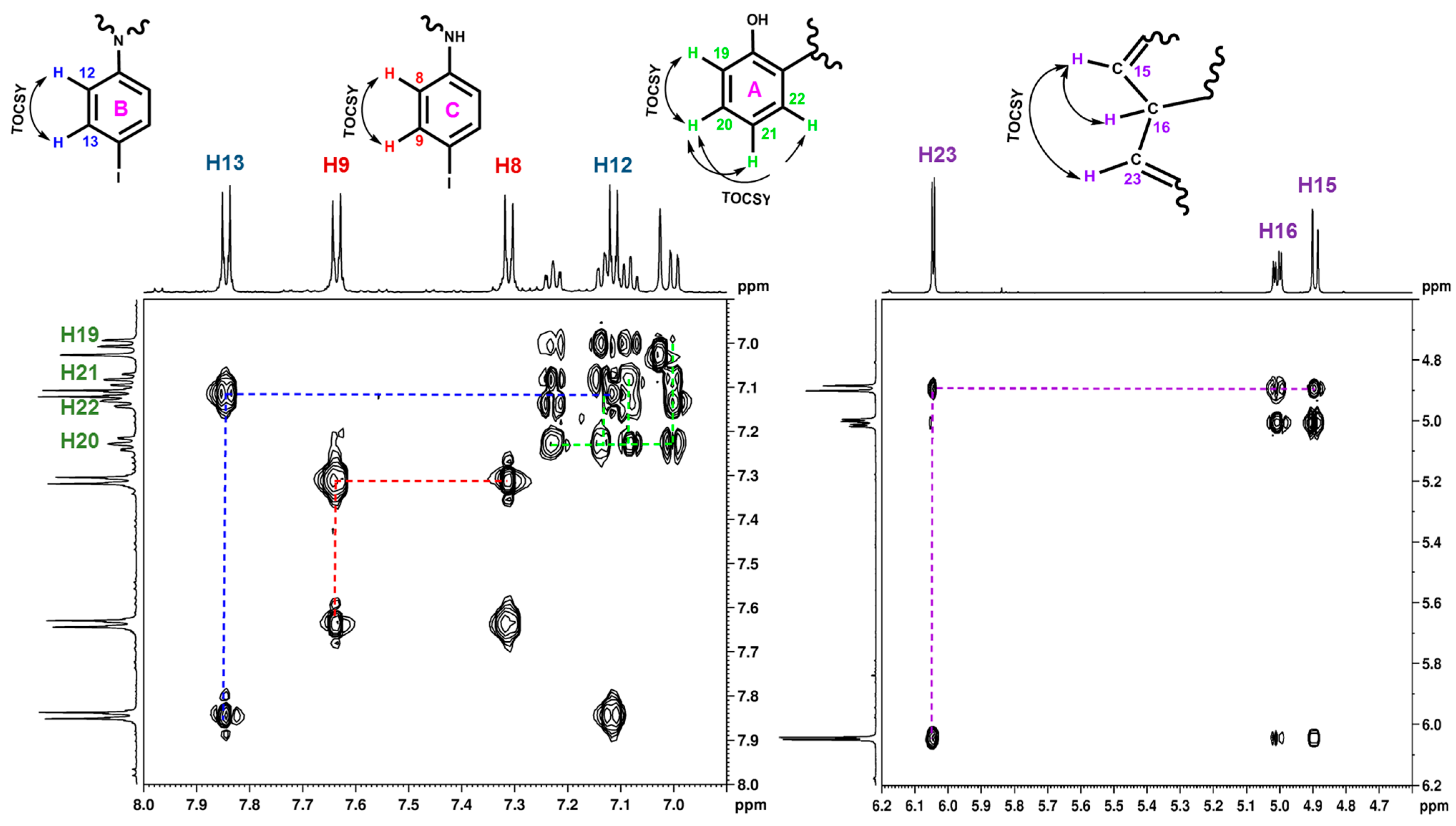

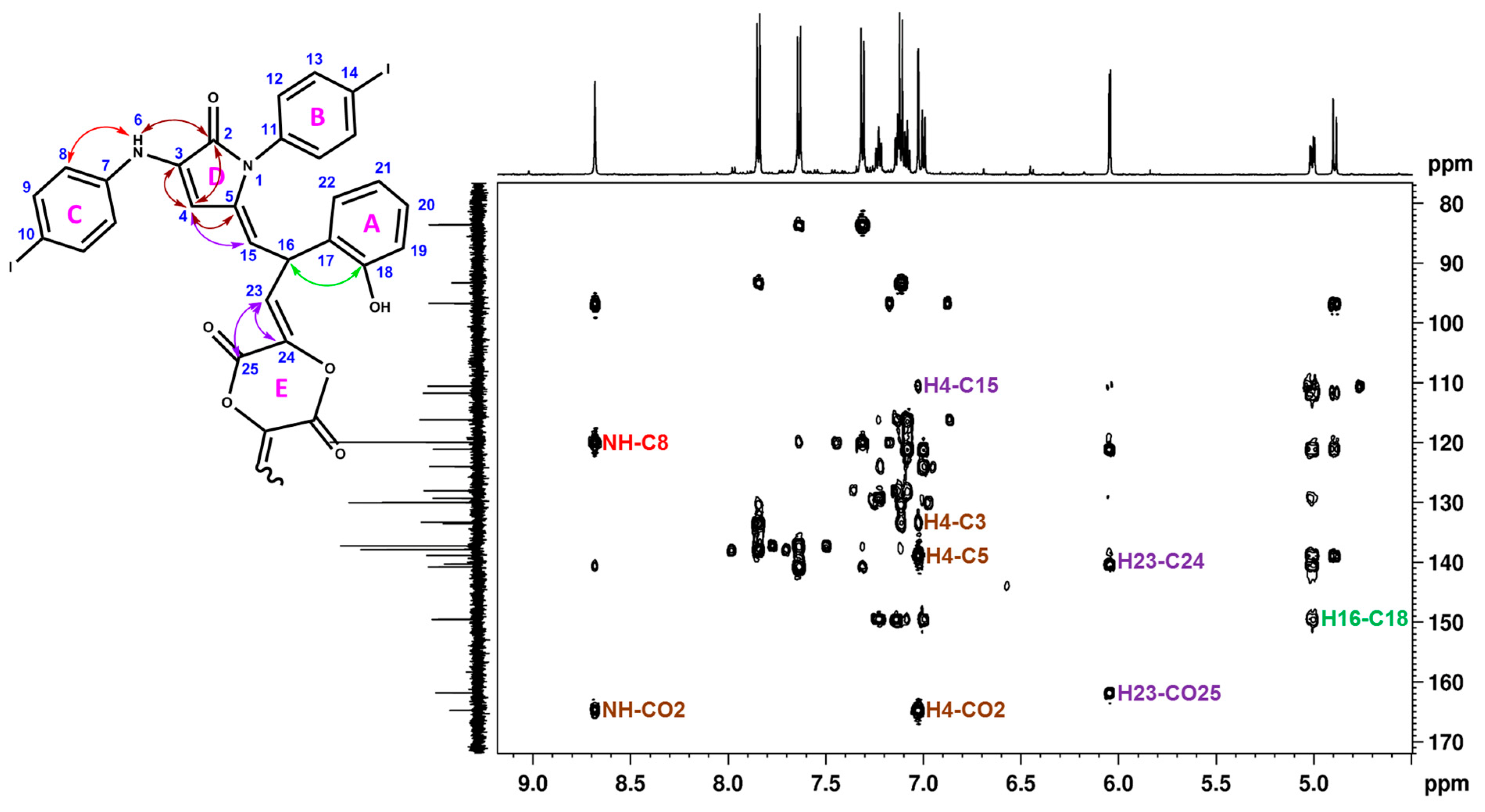

| HSQC Direct Correlations (Chemical Shift, ppm) | ||
| H16 (5.00)–C16 (31.8) | H19 (7.00)–C19 (116.2) | H22 (7.14)–C22 (129.3) |
| H4 (7.03)–C4 (96.7) | H8 (7.31)–C8 (120.0) | H12 (7.11)–C12 (130.0) |
| H15 (4.89)–C15 (110.6) | H21 (7.08)–C21 (124.0) | H9 (7.63)–C9 (137.3) |
| H23 (6.05)–C23 (111.7) | H20 (7.23)–C20 (128.1) | H13 (7.84)–C13 (137.9) |
| HMBC long range correlations | ||
| C16 (31.8)–H15, H22 | C8 (120.0)–NH | C3 (133.6)–H4 |
| C10 (83.6)–H8, H9 | C17 (121.1)–H15, H16, H19, H21, H23 | C5 (138.9)–H4, H15, H16 |
| C14 (93.3)–H12, H13 | C24 (140.3)–H16, H23 | |
| C4 (96.7)–H15, NH | C21 (124.0)–H19 | C7 (140.8)–H9 |
| C15 (110.6)–H16, H4 | C20 (128.1)–H22 | C18 (149.6)–H19, H20, H22 |
| C23 (111.7)–H15, H16 | C22 (129.3)–H20, H16 | C25 (161.8)–H23 |
| C19 (116.2)–H21 | C11 (133.3)–H13 | C2 (164.7)–H4, NH |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Matarneh, C.M.; Nicolescu, A. Structural Elucidation of a New Puzzling Compound Emerged from Doebner Quinoline Synthesis. Molbank 2024, 2024, M1841. https://doi.org/10.3390/M1841
Al-Matarneh CM, Nicolescu A. Structural Elucidation of a New Puzzling Compound Emerged from Doebner Quinoline Synthesis. Molbank. 2024; 2024(3):M1841. https://doi.org/10.3390/M1841
Chicago/Turabian StyleAl-Matarneh, Cristina Maria, and Alina Nicolescu. 2024. "Structural Elucidation of a New Puzzling Compound Emerged from Doebner Quinoline Synthesis" Molbank 2024, no. 3: M1841. https://doi.org/10.3390/M1841






