(Z)-N-Carbamoyl-4-hydroxy-4-(4-methoxyphenyl)-2-oxobut-3-enamide
Abstract
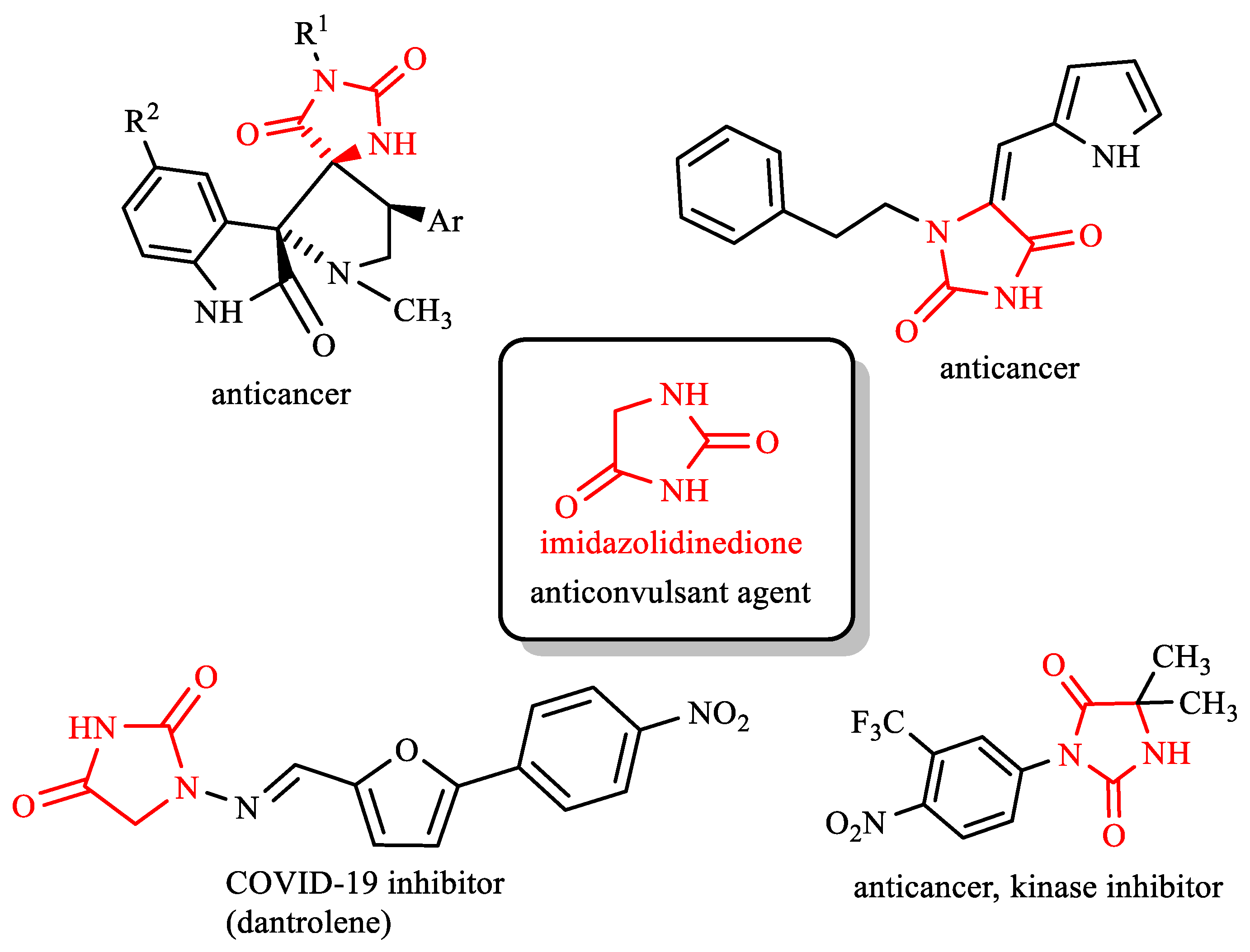
:1. Introduction
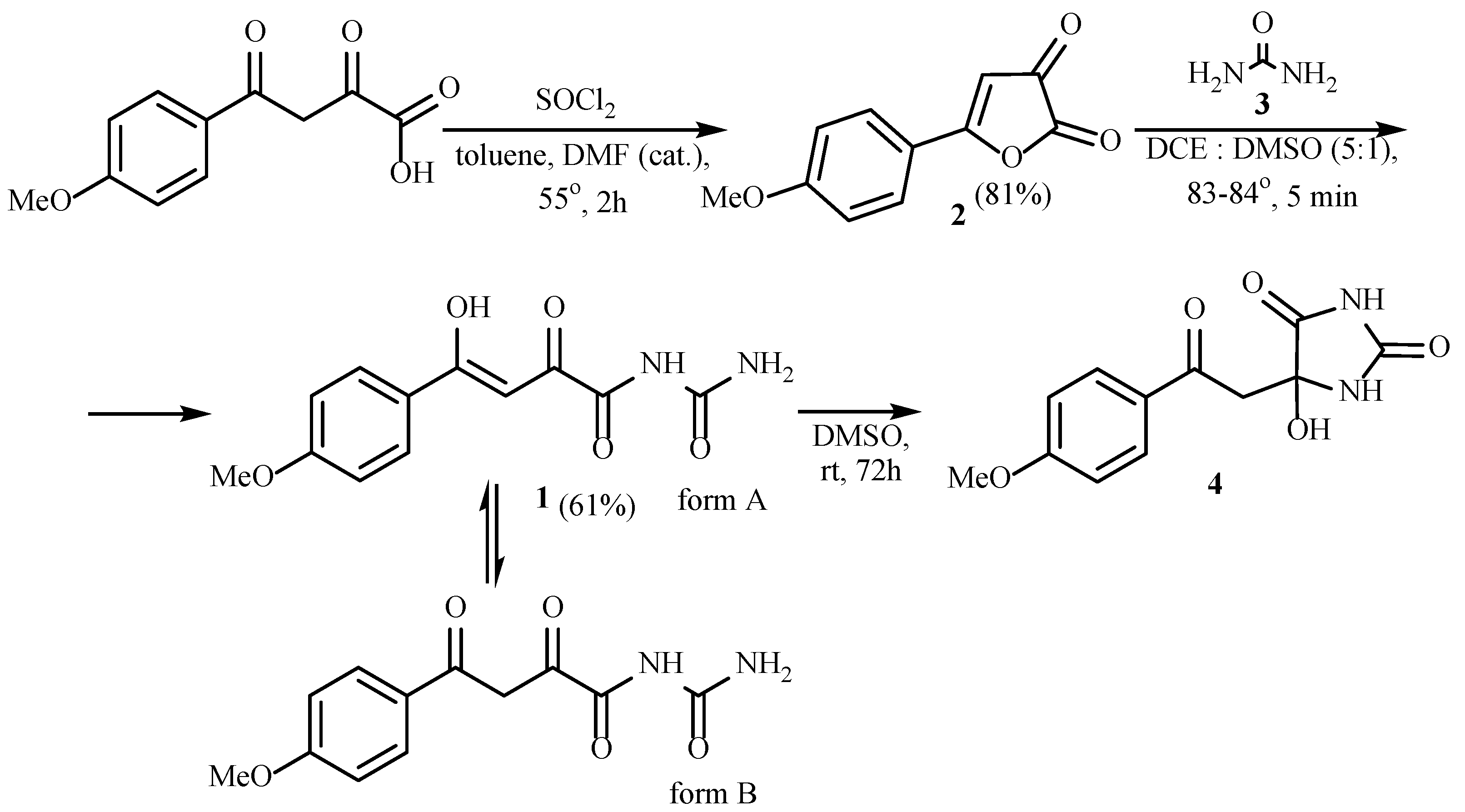
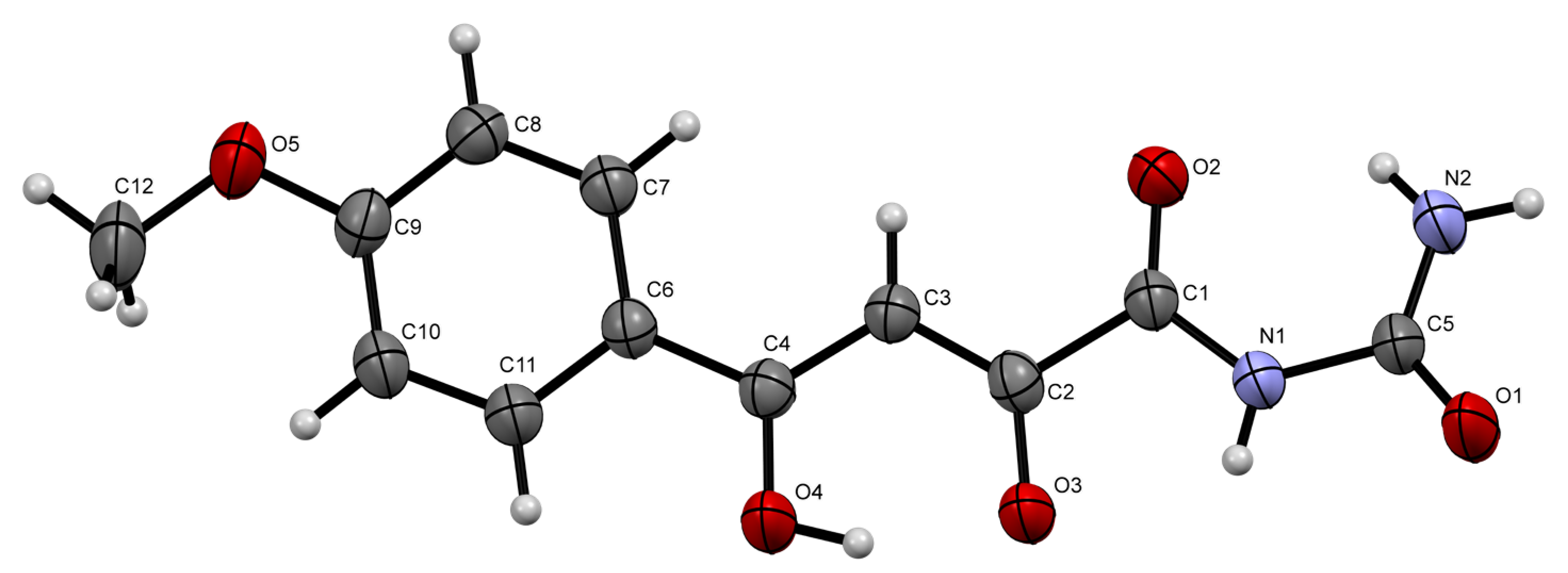
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. (Z)-N-Carbamoyl-4-hydroxy-4-(4-methoxyphenyl)-2-oxobut-3-enamide 1
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chahat; Bhatia, R.; Kumar, B. p53 as a potential target for treatment of cancer: A perspective on recent advancements in small molecules with structural insights and SAR studies. Eur. J. Med. Chem. 2023, 247, 115020. [Google Scholar] [CrossRef] [PubMed]
- Chaurasiya, A.; Sahu, C.; Wahan, S.K.; Chawla, P.A. Targeting cancer through recently developed purine clubbed heterocyclic scaffolds: An overview. J. Mol. Struct. 2023, 1280, 134967. [Google Scholar] [CrossRef]
- Jerin, S.; Harvey, A.J.; Lewis, A. Therapeutic Potential of Protein Tyrosine Kinase 6 in Colorectal Cancer. Cancers 2023, 15, 3703. [Google Scholar] [CrossRef] [PubMed]
- Naufal, M.; Hermawati, E.; Syah, Y.M.; Hidayat, A.T.; Hidayat, I.W.; Al-Anshori, J. Structure–Activity Relationship Study and Design Strategies of Hydantoin, Thiazolidinedione, and Rhodanine-Based Kinase Inhibitors: A Two-Decade Review. ACS Omega 2024, 9, 4186–4209. [Google Scholar] [CrossRef] [PubMed]
- Alkazmi, L.; Al-kuraishy, H.M.; Al-Gareeb, A.I.; El-Bouseary, M.M.; Ahmed, E.A.; Batiha, G.E.S. Dantrolene and ryanodine receptors in COVID-19: The daunting task and neglected warden. Clin. Exp. Pharmacol. Physiol. 2023, 50, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Gao, S.; Ye, B.; Yang, M.; Cheng, Y.; Kang, D.; Yi, F.; Sun, J.-P.; Menéndez-Arias, L.; Neyts, J.; et al. Medicinal chemistry strategies towards the development of non-covalent SARS-CoV-2 Mpro inhibitors. Acta Pharm. Sin. B 2024, 14, 87–109. [Google Scholar] [CrossRef] [PubMed]
- Abd-Allah, W.H.; El-Mohsen Anwar, M.A.; Mohammed, E.R.; El Moghazy, S.M. Anticonvulsant Classes and Possible Mechanism of Actions. ACS Chem. Neurosci. 2023, 14, 4076–4092. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, E.E.; Lukmanova, D.N.; Kasatkina, S.O.; Dmitriev, M.V.; Maslivets, A.N. Facile Synthesis of Regioisomeric N-Alkyl Substituted 3-Methylene-3,4-dihydroquinoxalin-2(1H)-ones. ChemistrySelect 2019, 4, 12774–12778. [Google Scholar] [CrossRef]
- Stepanova, E.E.; Dmitriev, M.V.; Maslivets, A.N. Synthesis of 1,4-benzothiazinones from acylpyruvic acids or furan-2,3-diones and o-aminothiophenol. Beilstein J. Org. Chem. 2020, 16, 2322–2331. [Google Scholar] [CrossRef] [PubMed]
- Dmitriev, M.V.; Khramtsova, E.E.; Apuskin, D.Y.; Andreev, A.I.; Kovalenko, I.I.; Mashevskaya, I.V.; Maslivets, A.N. Synthesis and Anti-Inflammatory Activity of (Z)-4-(2-(3-Oxopiperazin-2-ylidene)acetyl)benzoic Acid. Molbank 2024, 2024, M1772. [Google Scholar] [CrossRef]
- Stepanova, E.E.; Balandina, S.Y.; Drobkova, V.A.; Dmitriev, M.V.; Mashevskaya, I.V.; Maslivets, A.N. Synthesis, in vitro antibacterial activity against Mycobacterium tuberculosis, and reverse docking-based target fishing of 1,4-benzoxazin-2-one derivatives. Arch. Pharm. 2021, 354, e2000199. [Google Scholar] [CrossRef] [PubMed]
- Andreichikov, Y.S.; Nekrasov, D.D.; Rudenko, M.A.; Nalimova, Y.A. Reaction of 5-aryl-2,3-dihydrofuran-2,3-diones with N-substituted ureas and their thio and seleno analogs. Chem. Heterocycl. Compd. 1988, 10, 1411–1413. [Google Scholar]
- CrysAlisPro, Version 1.171.42.74a. Rigaku Oxford Diffraction: Wroclaw, Poland. 2022. Available online: https://www.rigaku.com/products/crystallography/crysalis (accessed on 5 April 2024).
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Andreychikov, Y.S. Guidelines for Student Research Work: Methods of Synthesis of Biologically Active Heterocyclic Compounds; Perm University: Perm, Russia, 1988; p. 9. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derevnina, A.O.; Andreeva, A.A.; Maslivets, A.N. (Z)-N-Carbamoyl-4-hydroxy-4-(4-methoxyphenyl)-2-oxobut-3-enamide. Molbank 2024, 2024, M1844. https://doi.org/10.3390/M1844
Derevnina AO, Andreeva AA, Maslivets AN. (Z)-N-Carbamoyl-4-hydroxy-4-(4-methoxyphenyl)-2-oxobut-3-enamide. Molbank. 2024; 2024(3):M1844. https://doi.org/10.3390/M1844
Chicago/Turabian StyleDerevnina, Alexandra O., Anastasia A. Andreeva, and Andrey N. Maslivets. 2024. "(Z)-N-Carbamoyl-4-hydroxy-4-(4-methoxyphenyl)-2-oxobut-3-enamide" Molbank 2024, no. 3: M1844. https://doi.org/10.3390/M1844
APA StyleDerevnina, A. O., Andreeva, A. A., & Maslivets, A. N. (2024). (Z)-N-Carbamoyl-4-hydroxy-4-(4-methoxyphenyl)-2-oxobut-3-enamide. Molbank, 2024(3), M1844. https://doi.org/10.3390/M1844






