Methyl and Benzyl (Ethyl 3,4-di-O-benzyl-2-O-benzoyl-1-thio-β-d-glucopyranosyl)uronate
Abstract
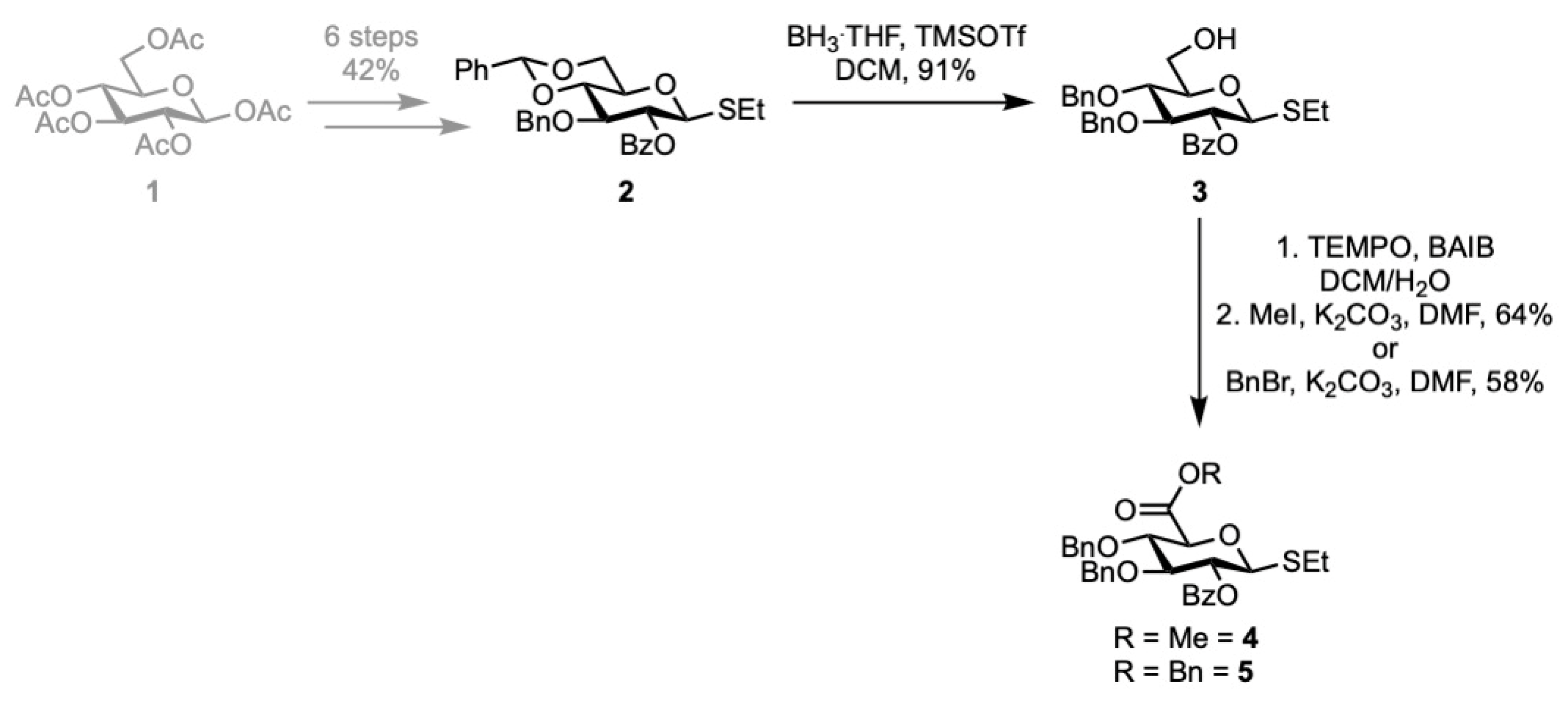
1. Introduction
2. Results
3. Materials and Methods
3.1. General
3.2. Ethyl 3,4-O-benzyl-2-O-benzoyl-1-thio-β-D-glucopyranoside (3)
3.3. Methyl (Ethyl 3,4-O-benzyl-2-O-benzoyl-1-thio-β-D-glucopyranoside)uronate (4)
3.4. Benzyl (Ethyl 3,4-O-benzyl-2-O-benzoyl-1-thio-β-D-glucopyranoside)uronate (5)
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pongener, I.; O’Shea, C.; Wootton, H.S.; Watkinson, M.; Miller, G.J. Developments in the Chemical Synthesis of Heparin and Heparan Sulfate. Chem. Rec. 2021, 21, 3238–3255. [Google Scholar] [CrossRef] [PubMed]
- Mende, M.; Bednarek, C.; Wawryszyn, M.; Sauter, P.; Biskup, M.B.; Schepers, U.; Bräse, S. Chemical Synthesis of Glycosaminoglycans. Chem. Rev. 2016, 116, 8193–8255. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.; Makshakova, O.; Angulo, J.; Bedini, E.; Bisio, A.; de Paz, J.L.; Fadda, E.; Guerrini, M.; Hricovini, M.; Lisacek, F.; et al. Glycosaminoglycans: What Remains to Be Deciphered? J. Am. Chem. Soc. Au 2023, 3, 628–656. [Google Scholar] [CrossRef] [PubMed]
- Pongener, I.; Miller, G.J. D-Glucuronate and D-Glucuronate Glycal Acceptors for the Scalable Synthesis of D-GlcN-α-1,4-D-GlcA Disaccharides and Modular Assembly of Heparan Sulfate. J. Org. Chem. 2023, 88, 11130–11139. [Google Scholar] [CrossRef] [PubMed]
- Pongener, I.; Sletten, E.T.; Danglad-Flores, J.; Seeberger, P.H.; Miller, G.J. Synthesis of a heparan sulfate tetrasaccharide using automated glycan assembly. Org. Biomol. Chem. 2024, 22, 1395–1399. [Google Scholar] [CrossRef] [PubMed]
- Meneghetti, M.C.Z.; Naughton, L.; O’shea, C.; Koffi Teki, D.S.E.; Chagnault, V.; Nader, H.B.; Rudd, T.R.; Yates, E.A.; Kovensky, J.; Miller, G.J.; et al. Using NMR to Dissect the Chemical Space and O-Sulfation Effects within the O- A nd S-Glycoside Analogues of Heparan Sulfate. ACS Omega 2022, 7, 24461–24467. [Google Scholar] [CrossRef] [PubMed]
- Baráth, M.; Hansen, S.U.; Dalton, C.E.; Jayson, G.C.; Miller, G.J.; Gardiner, J.M. Modular synthesis of heparin-related tetra-, hexa- and octasaccharides with differential O-6 protections: Programming for regiodefined 6-O-modifications. Molecules 2015, 20, 6167–6180. [Google Scholar] [CrossRef] [PubMed]
- Mycroft-West, C.J.; Devlin, A.J.; Cooper, L.C.; Procter, P.; Miller, G.J.; Fernig, D.G.; Guerrini, M.; Guimond, S.E.; Lima, M.A.; Yates, E.A.; et al. Inhibition of BACE1, the β-secretase implicated in Alzheimer’s disease, by a chondroitin sulfate extract from Sardina pilchardus. Neural Regen. Res. 2020, 15, 1546–1553. [Google Scholar] [PubMed]
- Van Den Bos, L.J.; Codée, J.D.C.; Van Der Toorn, J.C.; Boltje, T.J.; Van Boom, J.H.; Overkleeft, H.S.; Van Der Marel, G.A. Thioglycuronides: Synthesis and Application in the Assembly of Acidic Oligosaccharides. Org. Lett. 2004, 6, 2165–2168. [Google Scholar] [CrossRef] [PubMed]
- De Mico, A.; Margarita, R.; Parlanti, L.; Vescovi, A.; Piancatelli, G. A Versatile and Highly Selective Hypervalent Iodine (III)/2,2,6,6-Tetramethyl-1-piperidinyloxyl-Mediated Oxidation of Alcohols to Carbonyl Compounds. J. Org. Chem. 1997, 62, 6974–6977. [Google Scholar] [CrossRef]
- Van Den Bos, L.J.; Codée, J.D.C.; Litjens, R.E.J.N.; Dinkelaar, J.; Overkleeft, H.S.; Van Der Marel, G.A. Uronic acids in oligosaccharide synthesis. Eur. J. Org. Chem. 2007, 24, 3963–3976. [Google Scholar] [CrossRef]
- Zhu, Y.; Tyrikos-Ergas, T.; Schiefelbein, K.; Grafmüller, A.; Seeberger, P.H.; Delbianco, M. Automated access to well-defined ionic oligosaccharides. Org. Biomol. Chem. 2020, 18, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Daragics, K.; Fügedi, P. Synthesis of glycosaminoglycan oligosaccharides. Part 5: Synthesis of a putative heparan sulfate tetrasaccharide antigen involved in prion diseases. Tetrahedron 2010, 66, 8036–8046. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wootton, H.S.; Miller, G.J. Methyl and Benzyl (Ethyl 3,4-di-O-benzyl-2-O-benzoyl-1-thio-β-d-glucopyranosyl)uronate. Molbank 2024, 2024, M1847. https://doi.org/10.3390/M1847
Wootton HS, Miller GJ. Methyl and Benzyl (Ethyl 3,4-di-O-benzyl-2-O-benzoyl-1-thio-β-d-glucopyranosyl)uronate. Molbank. 2024; 2024(3):M1847. https://doi.org/10.3390/M1847
Chicago/Turabian StyleWootton, Hannah S., and Gavin J. Miller. 2024. "Methyl and Benzyl (Ethyl 3,4-di-O-benzyl-2-O-benzoyl-1-thio-β-d-glucopyranosyl)uronate" Molbank 2024, no. 3: M1847. https://doi.org/10.3390/M1847
APA StyleWootton, H. S., & Miller, G. J. (2024). Methyl and Benzyl (Ethyl 3,4-di-O-benzyl-2-O-benzoyl-1-thio-β-d-glucopyranosyl)uronate. Molbank, 2024(3), M1847. https://doi.org/10.3390/M1847






