Synthesis of Pyridinium Moiety Containing Triazolyl Purines
Abstract
:1. Introduction
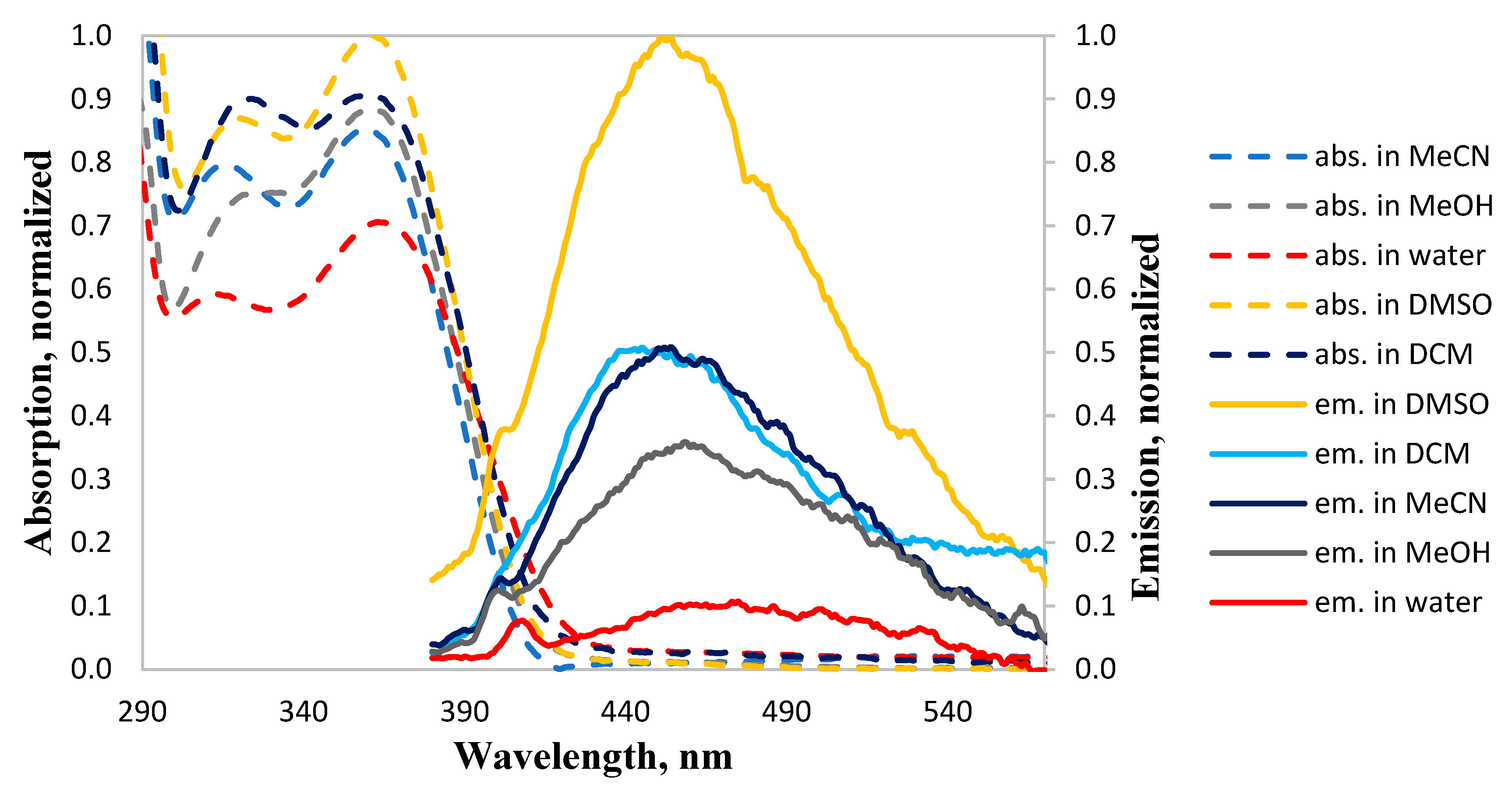
2. Results and Discussion
3. Materials and Method
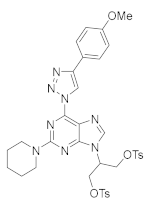
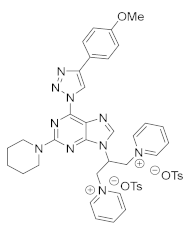
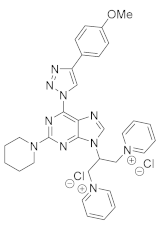
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seley-Radtke, K.L.; Yates, M.K. The evolution of nucleoside analogue antivirals: A review for chemists and non-chemists. Part 1: Early structural modifications to the nucleoside scaffold. Antivir. Res. 2018, 154, 66–86. [Google Scholar] [CrossRef] [PubMed]
- Legraverend, M. Recent advances in the synthesis of purine derivatives and their precursors. Tetrahedron 2008, 64, 8585–8603. [Google Scholar] [CrossRef]
- Shaw, G. 4.09-Purines. In Comprehensive Heterocyclic Chemistry; Katritzky, A.R., Rees, C.W., Eds.; Elsevier: Pergamon, Turkey, 1984; Volume 5, pp. 499–605. [Google Scholar]
- Joule, J.A.; Mills, K. Purines. In Heterocyclic Chemistry at a Glance; John Wiley & Sons, Ltd.: Chichester, UK, 2012; pp. 122–131. [Google Scholar]
- Hocek, M. Syntheses of purines bearing carbon substituents in positions 2, 6 or 8 by metal- or organometal-mediated C-C bond-forming reactions. Eur. J. Org. Chem. 2003, 2003, 245–254. [Google Scholar] [CrossRef]
- Manvar, A.; Shah, A. Microwave-assisted chemistry of purines and xanthines. An overview Dedicated to the late Professor V.M. Thakor on his 94th birthday. Tetrahedron 2013, 69, 8105–8127. [Google Scholar] [CrossRef]
- Novosjolova, I.; Bizdēna, Ē.; Turks, M. Synthesis and applications of azolylpurine and azolylpurine nucleoside derivatives. Eur. J. Org. Chem. 2015, 2015, 3629–3649. [Google Scholar] [CrossRef]
- Kovaļovs, A.; Novosjolova, I.; Bizdēna, Ē.; Bižane, I.; Skardziute, L.; Kazlauskas, K.; Jursenas, S.; Turks, M. 1,2,3-Triazoles as leaving groups in purine chemistry: A three-step synthesis of N6-substituted-2-triazolyl-adenine nucleosides and photophysical properties thereof. Tetrahedron Lett. 2013, 54, 850–853. [Google Scholar] [CrossRef]
- Zaķis, J.M.; Ozols, K.; Novosjolova, I.; Vilšķērsts, R.; Mishnev, A.; Turks, M. Sulfonyl group dance: A tool for the synthesis of 6-azido-2-sulfonylpurine derivatives. J. Org. Chem. 2020, 85, 4753–4771. [Google Scholar] [CrossRef] [PubMed]
- Legraverend, M.; Grierson, D.S. The purines: Potent and versatile small molecule inhibitors and modulators of key biological targets. Bioorg. Med. Chem. 2006, 14, 3987–4006. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Singh, J.; Ojha, R.; Singh, H.; Kaur, M.; Bedi, P.M.S.; Nepali, K. Design strategies, structure-activity relationship and mechanistic insights for purines as kinase inhibitors. Eur. J. Med. Chem. 2016, 112, 298–346. [Google Scholar] [CrossRef]
- Matarazzo, A.; Brow, J.; Hudson, R.H.E. Synthesis and photophysical evaluation of new fluorescent 7-arylethynyl-7-deazaadenosine analogs. Can. J. Chem. 2018, 96, 1093–1100. [Google Scholar] [CrossRef]
- Saito, Y.; Hudson, R.H.E. Base-modified fluorescent purine nucleosides and nucleotides for use in oligonucleotide probes. J. Photochem. Photobiol. C Photochem. Rev. 2018, 36, 48–73. [Google Scholar] [CrossRef]
- Venkatesham, A.; Pillalamarri, S.R.; Wit, F.; Lescrinier, E.; Debyser, Z.; Aerschot, A. Propargylated purine deoxynucleosides: New tools for fluorescence imaging strategies. Molecules 2019, 24, 1–16. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zatorska, D.; Kim, J.; Aguirre, J.; Llauger, L.; She, Y.; Wu, N.; Immormino, R.M.; Gewirth, D.T.; Chiosis, G. Identification of potent water-soluble purine-scaffold inhibitors of the heat shock protein 90. J. Med. Chem. 2006, 49, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Gołdyn, M.R.; Larowska, D.; Bartoszak-Adamska, E. Novel Purine Alkaloid Cocrystals with Trimesic and Hemimellitic Acids as Coformers: Synthetic Approach and Supramolecular Analysis. Cryst. Growth Des. 2021, 21, 396–413. [Google Scholar] [CrossRef] [PubMed]
- Carreira, A.R.F.; Veloso, T.; Schaeffer, N.; Pereira, J.L.; Ventura, S.P.M.; Rizzi, C.; Plénet, J.S.; Passos, H.; Coutinho, J.A.P. Synthesis of purine-based ionic liquids and their applications. Molecules 2021, 26, 6958. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.M.; McLaughlin, C.K.; Lantero, D.R.; Manderville, R.A. Biomarkers for phenol carcinogen exposure act as pH-sensing fluorescent probes. J. Am. Chem. Soc. 2007, 129, 1894–1895. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mital, S. Electronic and photocatalytic properties of purine(s)-capped CdS nanoparticles in the presence of tryptophol. J. Mol. Catal. A Chem. 2004, 219, 65–71. [Google Scholar] [CrossRef]
- Jovaisaite, J.; Cīrule, D.; Jeminejs, A.; Novosjolova, I.; Turks, M.; Baronas, P.; Komskis, R.; Tumkevicius, S.; Jonusauskas, G.; Jursenas, S. Proof of principle of a purine D-A-D′ ligand based ratiometric chemical sensor harnessing complexation induced intermolecular PET. Phys. Chem. Chem. Phys. 2020, 22, 26502–26508. [Google Scholar] [CrossRef]
- Burcevs, A.; Sebris, A.; Traskovskis, K.; Chu, H.W.; Chang, H.T.; Jovaišaitė, J.; Juršėnas, S.; Turks, M.; Novosjolova, I. Synthesis of Fluorescent C–C Bonded Triazole-Purine Conjugates. J. Fluoresc. 2024, 34, 1091–1097. [Google Scholar] [CrossRef]
- Sebris, A.; Novosjolova, I.; Traskovskis, K.; Kokars, V.; Tetervenoka, N.; Vembris, A.; Turks, M. Photophysical and Electrical Properties of Highly Luminescent 2/6-Triazolyl-Substituted Push-Pull Purines. ACS Omega 2022, 7, 5242–5253. [Google Scholar] [CrossRef]
- Šišuļins, A.; Bucevičius, J.; Tseng, Y.T.; Novosjolova, I.; Traskovskis, K.; Bizdēna, Ē.; Chang, H.T.; Tumkevičius, S.; Turks, M. Synthesis and fluorescent properties of N(9)-alkylated 2-amino-6-triazolylpurines and 7-deazapurines. Beilstein J. Org. Chem. 2019, 15, 474–489. [Google Scholar] [CrossRef] [PubMed]
- Sajomsang, W.; Gonil, P.; Ruktanonchai, U.R.; Petchsangsai, M.; Opanasopit, P.; Puttipipatkhachorn, S. Effects of molecular weight and pyridinium moiety on water-soluble chitosan derivatives for mediated gene delivery. Carbohydr. Polym. 2013, 91, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.B.; Islam, M.I.; Nath, N.; Emran, T.B.; Rahman, M.R.; Sharma, R.; Matin, M.M. Recent Advances in Pyridine Scaffold: Focus on Chemistry, Synthesis, and Antibacterial Activities. BioMed Res. Int. 2023, 2023, 9967591. [Google Scholar] [CrossRef] [PubMed]
- Wade, D.A.; Tucker, S.A. Spectrochemical evaluation of pyridinium chloride as a possible selective fluorescence quenching agent of polycyclic aromatic hydrocarbons in water and neat acetonitrile. Talanta 2000, 53, 571–578. [Google Scholar] [CrossRef]
- Hann, R.A.; Rosseinsky, D.R.; White, T.P. Inter- and intra-molecular quenching of anthracene fluorescence by pyridinium ion in solution. J. Chem. Soc. Faraday Trans. 2 1974, 70, 1522–1525. [Google Scholar] [CrossRef]

| Solvent | Compound 3 | Compound 4 | ||||
|---|---|---|---|---|---|---|
| λabs max, nm | λem max, nm | QY, % | λabs max, nm | λem max, nm | QY, % | |
| MeCN | - | - | - | 360 | 457 | <0.5 |
| MeOH | - | - | - | 362 | 461 | <0.5 |
| H2O | 362 | 457 | <0.5 | 363 | 463 | <0.5 |
| DMSO | 361 | 428 | <0.5 | 362 | 454 | <0.5 |
| DCM | 359 | 565 | <0.5 | 360 | 452 | <0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burcevs, A.; Turks, M.; Novosjolova, I. Synthesis of Pyridinium Moiety Containing Triazolyl Purines. Molbank 2024, 2024, M1855. https://doi.org/10.3390/M1855
Burcevs A, Turks M, Novosjolova I. Synthesis of Pyridinium Moiety Containing Triazolyl Purines. Molbank. 2024; 2024(3):M1855. https://doi.org/10.3390/M1855
Chicago/Turabian StyleBurcevs, Aleksejs, Māris Turks, and Irina Novosjolova. 2024. "Synthesis of Pyridinium Moiety Containing Triazolyl Purines" Molbank 2024, no. 3: M1855. https://doi.org/10.3390/M1855






