(R)-2-Amino-1-hydroxyethylphosphonic Acid
Abstract
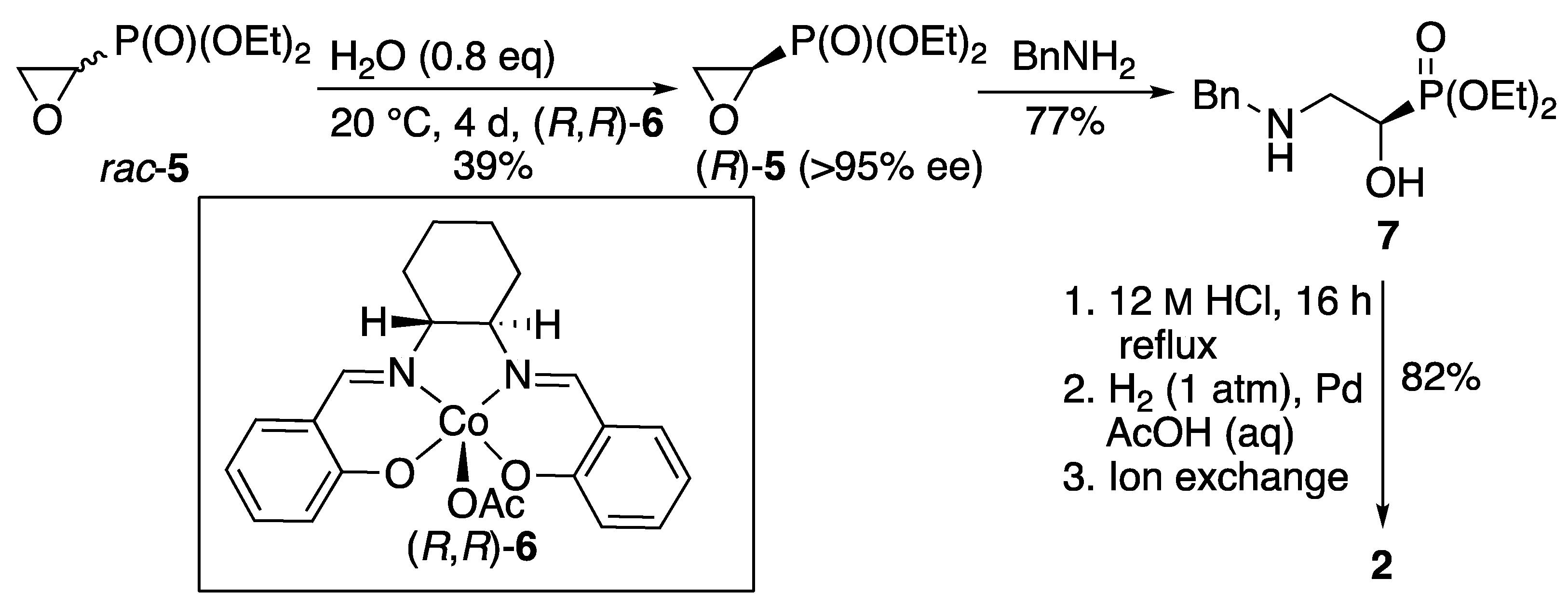
1. Introduction
2. Results
3. Experimental
3.1. General Experimental Details
3.2. (R)-Diethyl Oxiran-2-ylphosphonate (R)-5
3.3. (R)-Diethyl (2-benzylamino-1-hydroxyethyl)phosphonate 7
3.4. (R)-2-Amino-1-hydroxyethylphosphonic Acid 2
3.5. X-ray Structure Determination of 2
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duhamel, S.; Diaz, J.M.; Adams, J.C.; Djaoudi, K.; Steck, V.; Waggoner, E.M. Phosphorus as an integral component of global marine geochemistry. Nat. Geosc. 2021, 14, 359–368. [Google Scholar] [CrossRef]
- McSorley, F.R.; Wyatt, P.B.; Martinez, A.; DeLong, E.F.; Hove-Jensen, B.; Zechel, D.L. PhnY and PhnZ comprise a new oxidative pathway for enzymatic cleavage of a carbon-phosphorus bond. J. Am. Chem. Soc. 2012, 134, 8364–8367. [Google Scholar] [CrossRef] [PubMed]
- Van Staalduinen, L.M.; McSorley, F.R.; Schiessl, K.; Séguin, J.; Wyatt, P.B.; Hammerschmidt, F.; Zechel, D.L.; Jia, Z. Crystal structure of Phnz in complex with substrate reveals a di-iron oxygenase mechanism for catabolism of organophosphates. Proc. Natl. Acad. Sci. USA 2014, 111, 5171–5176. [Google Scholar] [CrossRef] [PubMed]
- Kolodiazhnyi, O.I.; Kolodiazhna, A.O. Stereoselective syntheses of organophosphorus compounds. Symmetry 2024, 16, 342. [Google Scholar] [CrossRef]
- Wyatt, P.B.; Blakskjær, P. An enantioselective synthesis of (R)-2-amino-1-hydroxyethylphosphonic acid by hydrolytic kinetic resolution of (±)-diethyl oxiranephosphonate. Tetrahedron Lett. 1999, 40, 6481–6483. [Google Scholar] [CrossRef]
- Tokunaga, M.; Larrow, J.F.; Kakiuchi, F.; Jacobsen, E.N. Asymmetric catalysis with water: Efficient kinetic resolution of terminal epoxides by means of catalytic hydrolysis. Science 1997, 277, 936–938. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmidt, F.; Völlenkle, H. Absolute Konfiguration der (2-Amino-1-hydroxyethyl)phosphonsäure aus Ancanthamoeba castellanii (Neff)—Darstellung der Phosphonsaure-Analoga von (+)- und (−)- Serin. Liebigs Ann. Chem. 1989, 577–583. [Google Scholar] [CrossRef]
- Hammerschmidt, F.; Lindner, W.; Wuggenig, F.; Zarble, E. Enzymes in organic chemistry. Part 10: Chemo-enzymatic synthesis of L-phosphaserine and L-phosphaisoserine and enantioseparation of amino-hydroxyethylphosphonic acids by non-aqueous capillary electrophoresis with quinine carbamate as chiral pair agent. Tetrahedron Asymmetry 2000, 11, 2955–2964. [Google Scholar] [CrossRef]
- Pallitsch, K.; Happl, B.; Stieger, C. Determination of the absolute configuration of (−)-hydroxynitralaphos and related biosynthetic questions. Chem. Eur. J. 2017, 23, 15655–15665. [Google Scholar] [CrossRef] [PubMed]
- Okaya, Y. Crystal structure of the stable modification of 2-aminoethylphosphonic acid, β-ciliatine. Acta Crystallogr. 1966, 20, 712–715. [Google Scholar] [CrossRef]
- Crawczyk, H.; Albrecht, L.; Wojciechowski, J.; Wolf, W.M. Synthesis and crystal structure of 1-(aminomethyl)vinylphosphonic acid. Tetrahedron 2008, 64, 5051–5054. [Google Scholar] [CrossRef]
- Chen, S.-P.; Zhang, Y.-Q.; Hu, L.; He, H.-Z.; Yuan, L.-J. Hydrogen-bonded assembly of aminophosphonic anions: Different 1D, 2D and 3D supramolecular architectures. CrystEngComm 2010, 12, 3327–3336. [Google Scholar] [CrossRef]
- Bogomilova, A.; Hägele, G.; Troev, K.; Wagner, E.; Günther, M. Hydrogen bonding in α-aminophosphonic acids. Phosphorus Sulfur Relat. Elem. 2012, 187, 165–180. [Google Scholar] [CrossRef]
- Wanat, W.; Dziuk, B.; Kafarski, P. New crystal structures of α-aminophosphonic acid analogues of phenylglycine. Struct. Chem. 2020, 31, 1197–1209. [Google Scholar] [CrossRef]
- Flack, H.D. On enantiomorph-polarity estimation. Acta. Crystallogr. 1983, A39, 876–881. [Google Scholar] [CrossRef]
- Sturtz, G.; Pondaven-Raphalen, A. Etude de la réaction de cyclisation des halohydrines-1,2-éthyl phosphonates de diéthyle pour l’obtention de l’époxy-1,2 éthyl phosphonate de diéthyle. Phosphorus Sulfur Relat. Elem. 1984, 20, 35–47. [Google Scholar] [CrossRef]

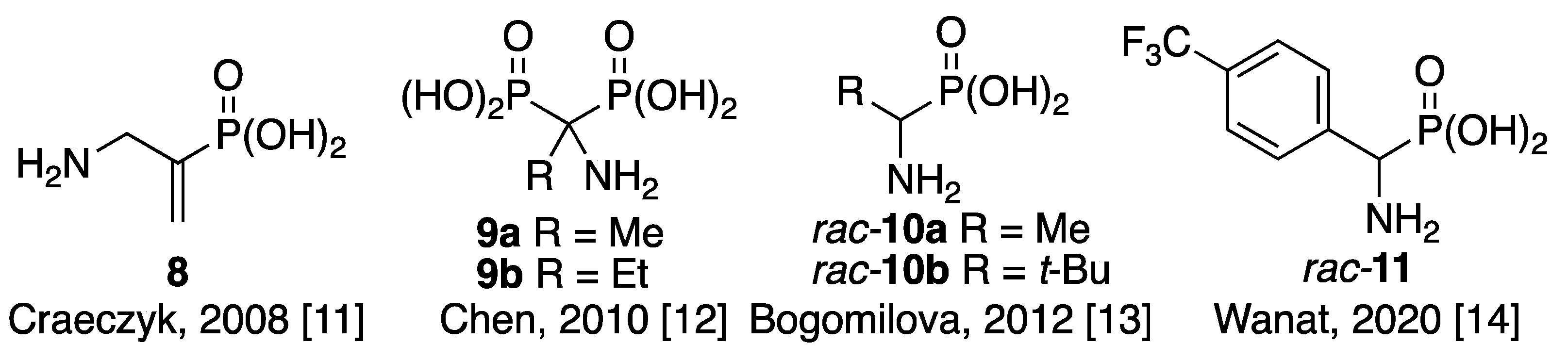

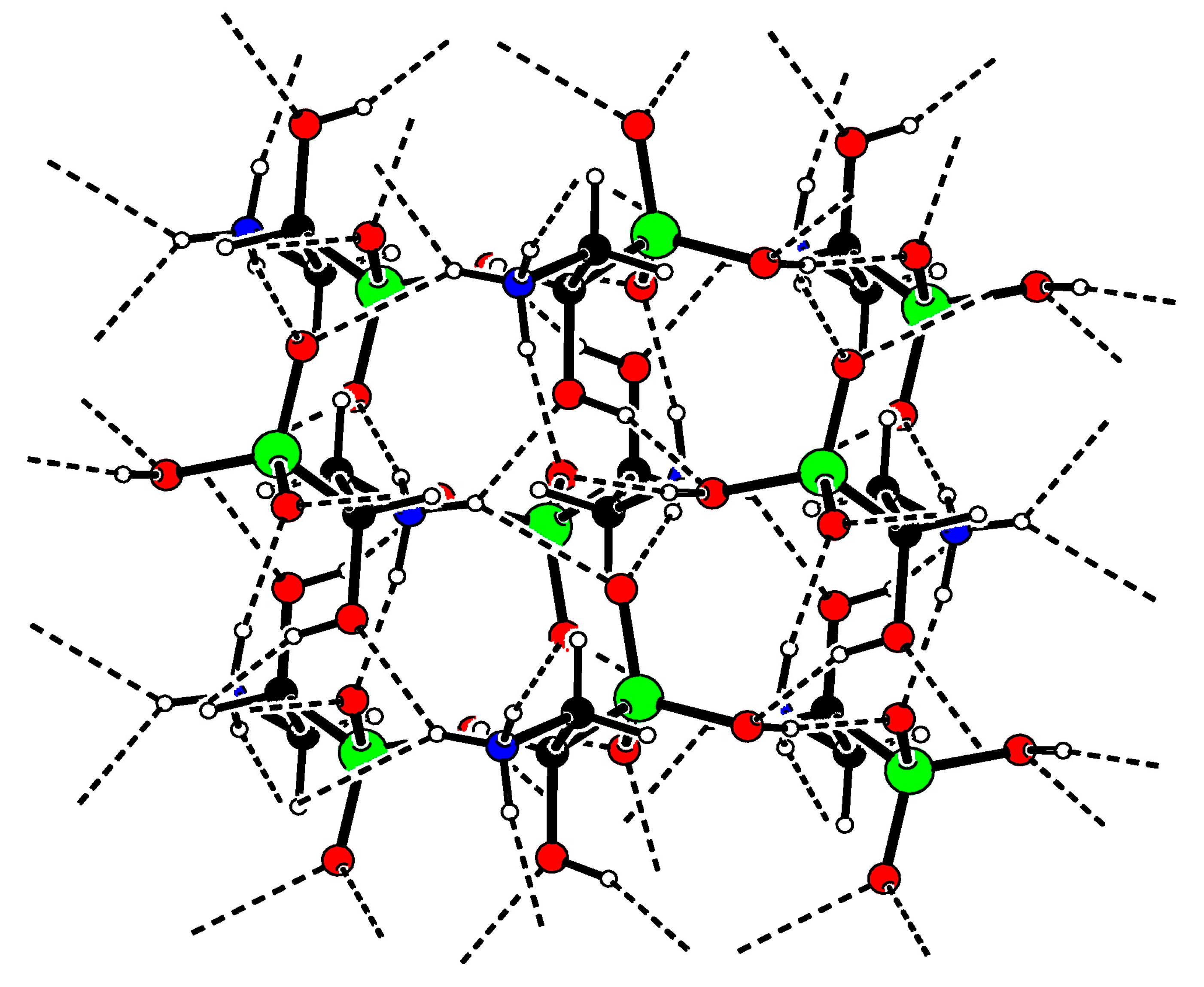
| Donor-H | Acceptor-H | Donor-Acceptor | Angle | |
|---|---|---|---|---|
| N1-H4•••O2 | 0.91 | 2.23 | 2.951 (2) | 135.5 |
| N1-H4•••O4 | 0.91 | 2.20 | 2.945 (1) | 139.1 |
| N1-H5•••O2 | 0.91 | 1.84 | 2.730 (1) | 165.6 |
| N1-H6•••O3 | 0.91 | 1.90 | 2.795 (1) | 165.9 |
| O1-H7•••O3 | 0.84 | 1.69 | 2.503 (1) | 163.6 |
| O4-H8•••O1 | 0.84 | 2.12 | 2.880 (1) | 150.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motevalli, M.; Abrahams, I.; Blakskjær, P.; Wyatt, C.E.; Wyatt, P.B. (R)-2-Amino-1-hydroxyethylphosphonic Acid. Molbank 2024, 2024, M1888. https://doi.org/10.3390/M1888
Motevalli M, Abrahams I, Blakskjær P, Wyatt CE, Wyatt PB. (R)-2-Amino-1-hydroxyethylphosphonic Acid. Molbank. 2024; 2024(4):M1888. https://doi.org/10.3390/M1888
Chicago/Turabian StyleMotevalli, Majid, Isaac Abrahams, Peter Blakskjær, Caroline E. Wyatt, and Peter B. Wyatt. 2024. "(R)-2-Amino-1-hydroxyethylphosphonic Acid" Molbank 2024, no. 4: M1888. https://doi.org/10.3390/M1888
APA StyleMotevalli, M., Abrahams, I., Blakskjær, P., Wyatt, C. E., & Wyatt, P. B. (2024). (R)-2-Amino-1-hydroxyethylphosphonic Acid. Molbank, 2024(4), M1888. https://doi.org/10.3390/M1888







