Bis [4,4′-(1,3-Phenylenebis(azanylylidene))-bis(3,6-di-tert-butyl-2-oxycyclohexa-2,5-dien-1-one)-bis(dimethylsulfoxide)nickel(II)]
Abstract
1. Introduction
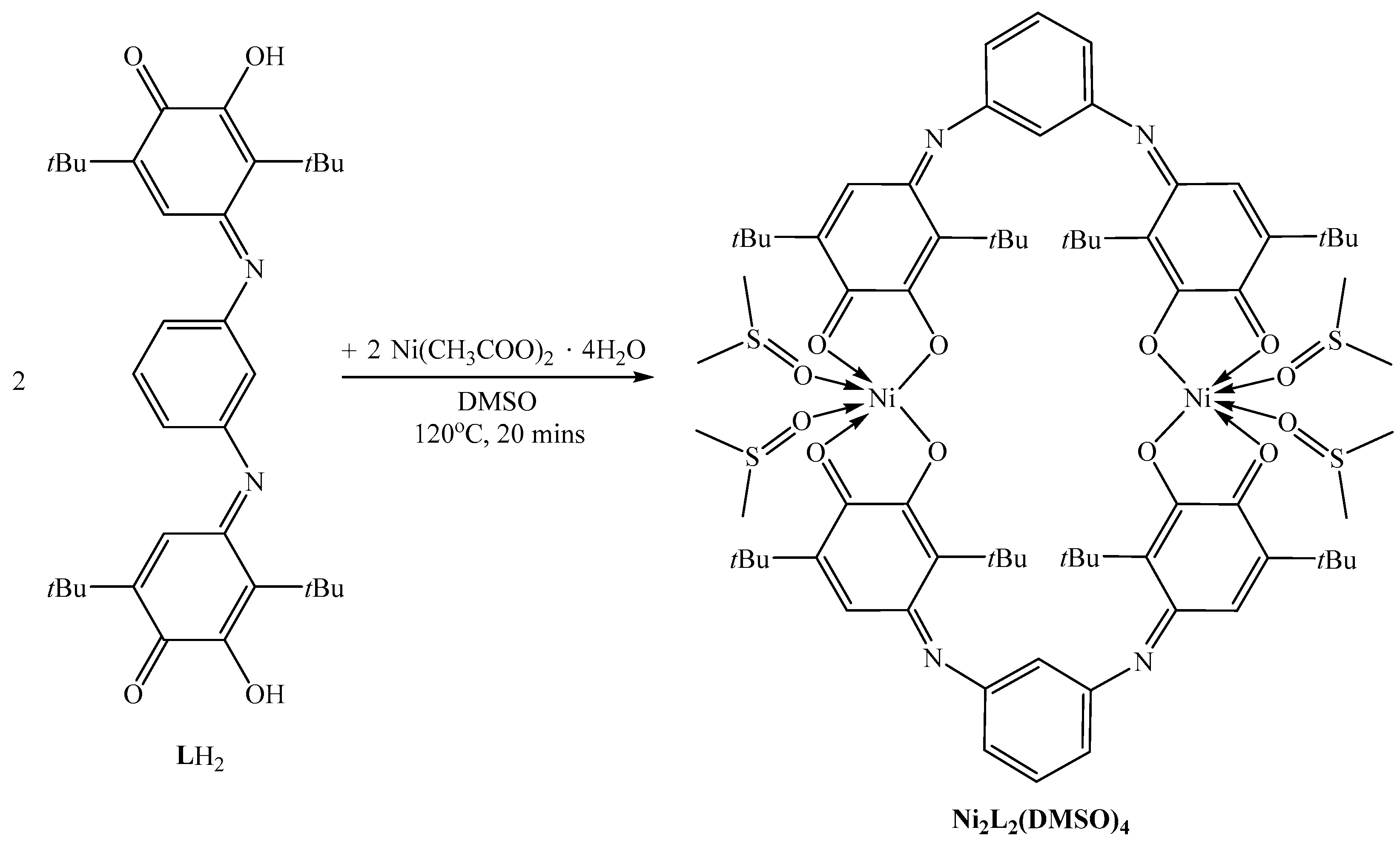
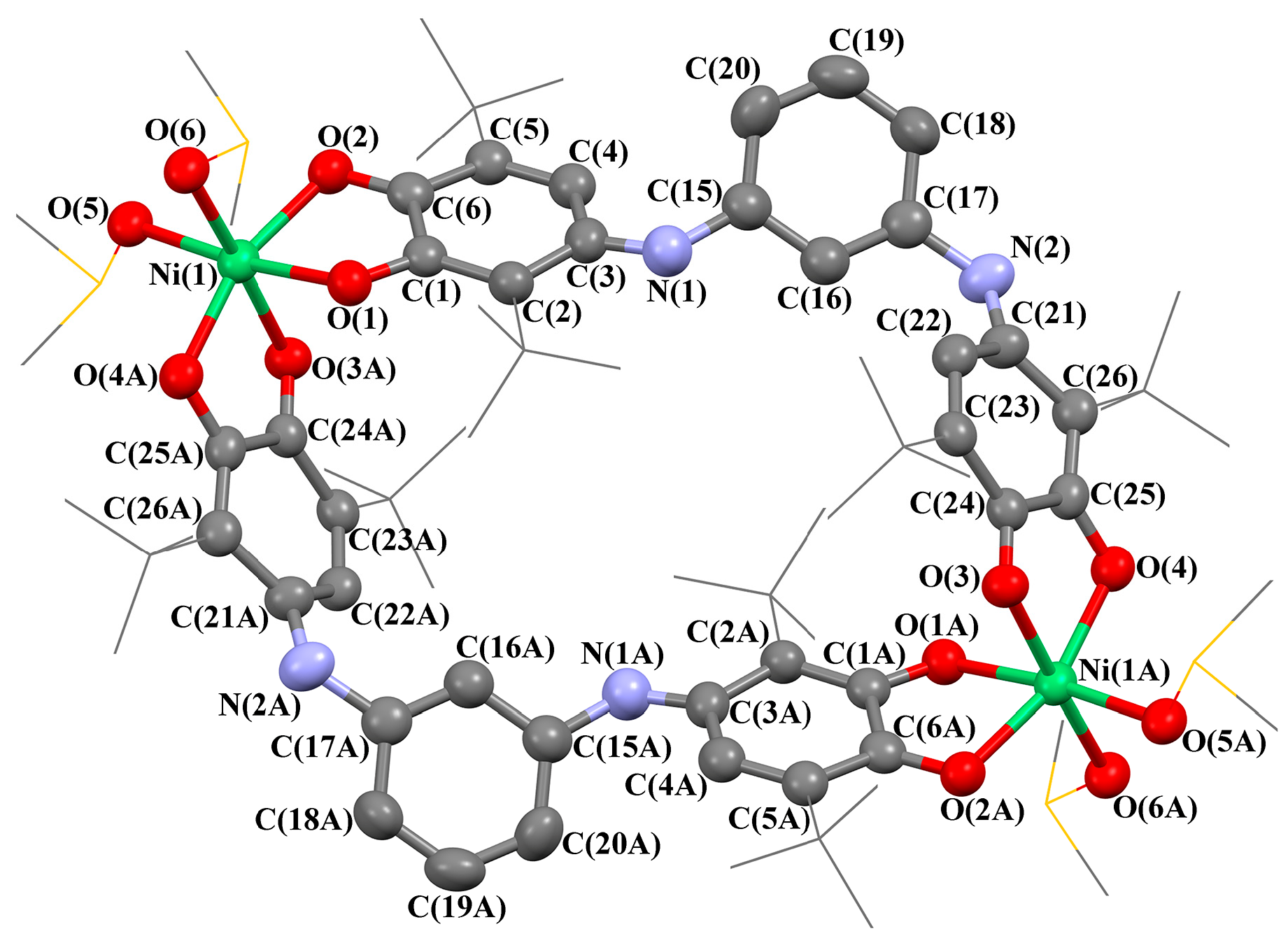
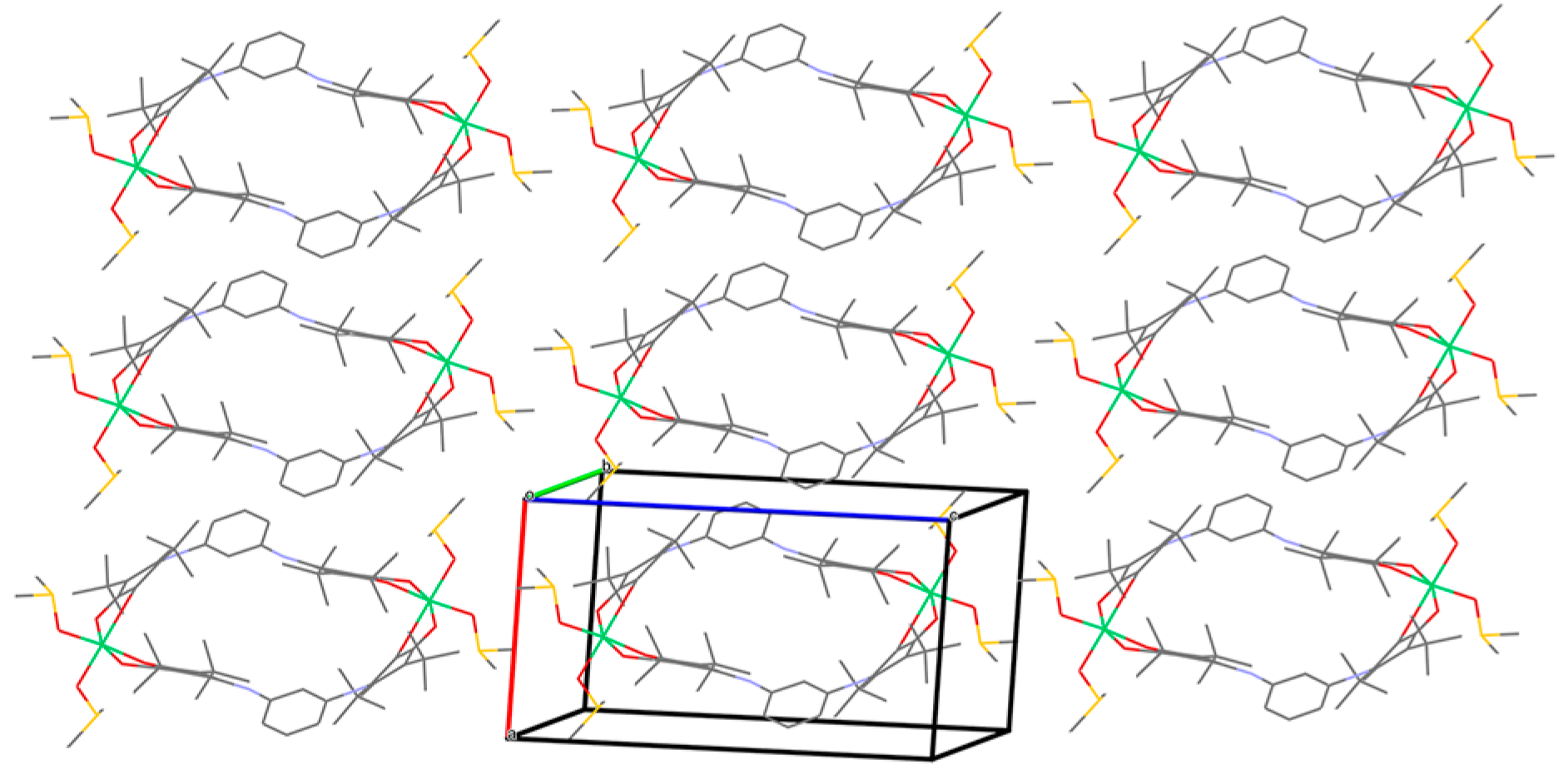
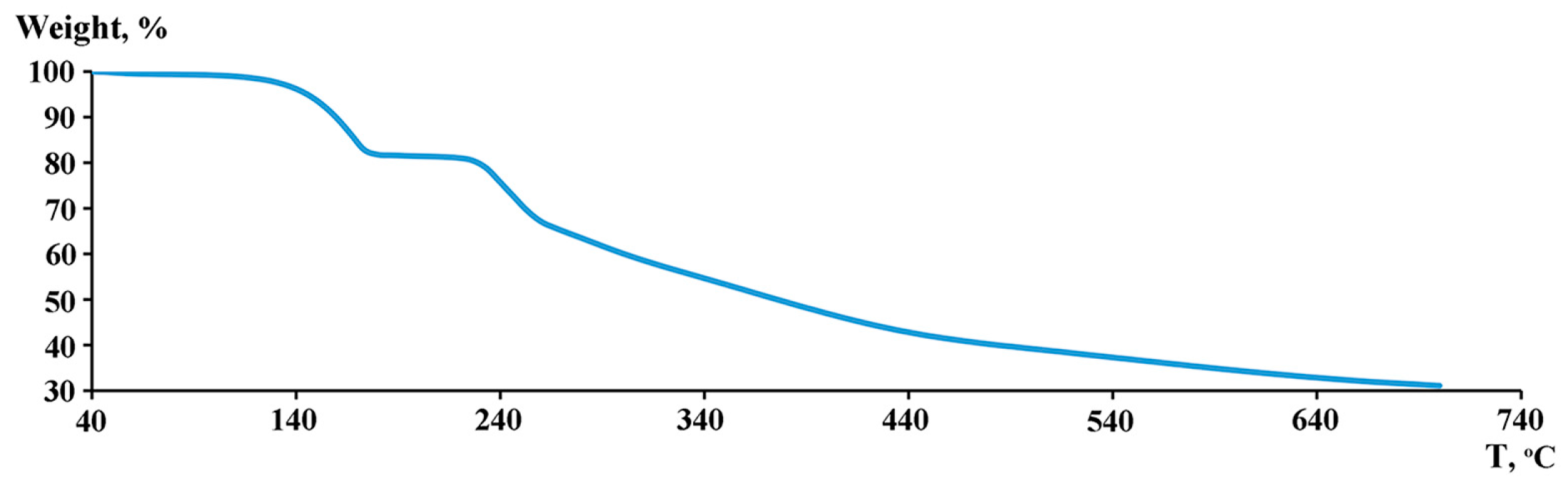
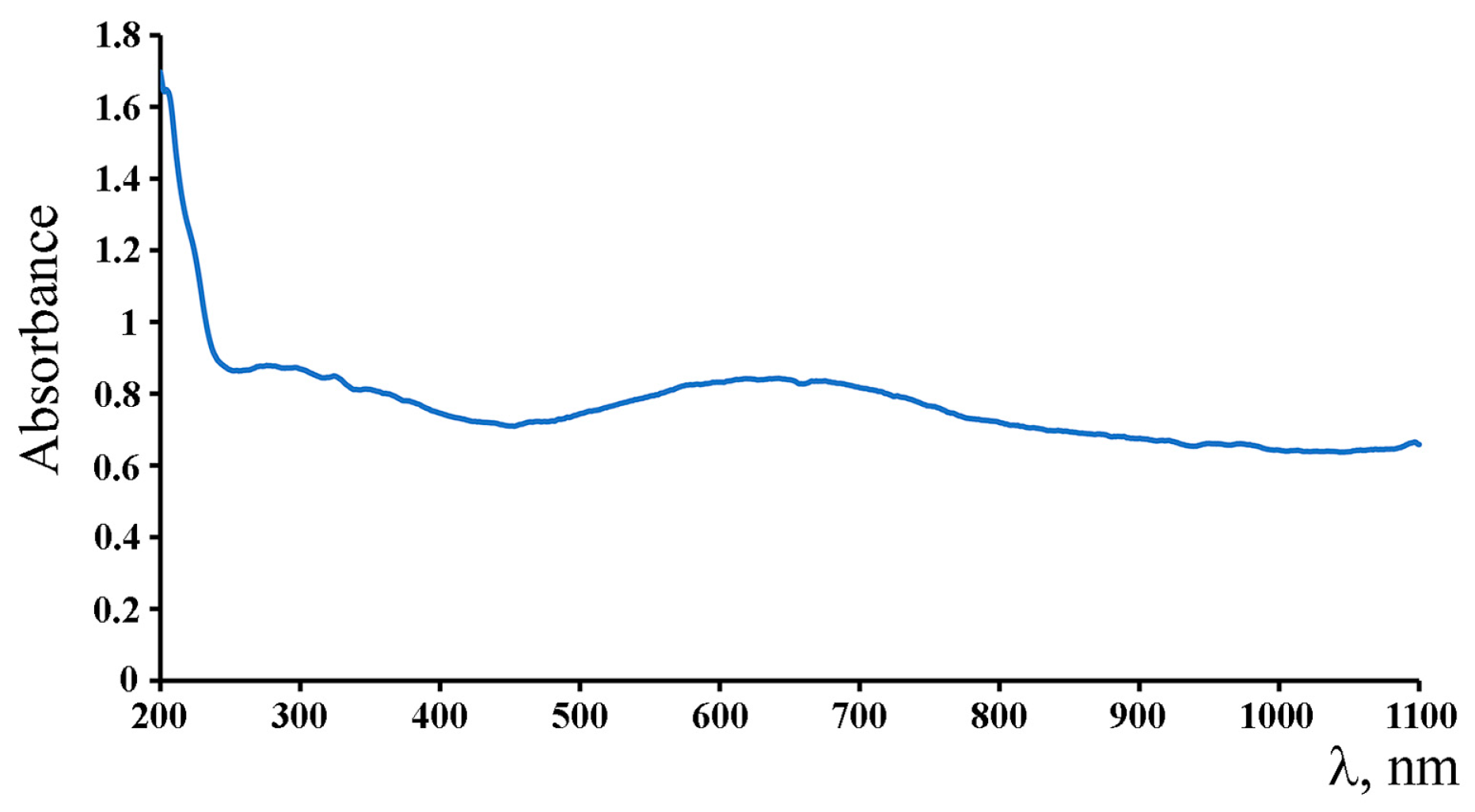
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis of Ni2L2(DMSO)4
3.3. Single-Crystal X-ray Diffraction Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Min, K.S.; DiPasquale, A.; Rheingold, A.L.; Miller, J.S. Room-Temperature Spin Crossover Observed for [(TPyA) FeII (DBQ2−) FeII (TPyA)]2+ [TPyA = Tris (2-pyridylmethyl) amine; DBQ2− = 2,5-Di-tert-butyl-3,6-dihydroxy-1,4-benzoquinonate]. Inorg. Chem. Com. 2007, 46, 1048–1050. [Google Scholar] [CrossRef] [PubMed]
- Min, K.S.; DiPasquale, A.G.; Rheingold, A.L.; White, H.S.; Miller, J.S. Observation of redox-induced electron transfer and spin crossover for dinuclear cobalt and iron complexes with the 2,5-di-tert-butyl-3,6-dihydroxy-1,4-benzoquinonate bridging ligand. J. Am. Chem. Soc. 2009, 131, 6229–6236. [Google Scholar] [CrossRef] [PubMed]
- Kanegawa, S.; Shiota, Y.; Kang, S.; Takahashi, K.; Okajima, H.; Sakamoto, A.; Iwata, T.; Kandori, H.; Yoshizawa, K.; Sato, O. Directional Electron Transfer in Crystals of [CrCo] Dinuclear Complexes Achieved by Chirality-Assisted Preparative Method. J. Am. Chem. Soc. 2016, 138, 14170–14173. [Google Scholar] [CrossRef] [PubMed]
- Simonson, A.N.; Kareis, C.M.; Ovanesyan, N.S.; Baumann, D.O.; Rheingold, A.L.; Arif, A.M.; Miller, J.S. Seven-coordinate tetraoxolate complexes. Polyhedron 2018, 139, 215–221. [Google Scholar] [CrossRef]
- Druzhkov, N.O.; Meshcheryakova, I.N.; Cherkasov, A.V.; Piskunov, A.V. New functionalized ditopic redox-active hydroxy-p-iminoquinone-type ligands and mercury(II) complexes based on these ligands. Russ. Chem. Bull. 2020, 69, 49–60. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kawata, S. Coordination compounds of 1,4-dihydroxybenzoquinone and its homologues. Structures and properties. Coord. Chemi. Rev. 2002, 224, 11–34. [Google Scholar] [CrossRef]
- D’Alessandro, D.M. Exploiting redox activity in metal–organic frameworks: Concepts, trends and perspectives. Chem. Commun. 2016, 52, 8957–8971. [Google Scholar] [CrossRef]
- Espallargas, G.M.; Coronado, E. Magnetic functionalities in MOFs: From the framework to the pore. Chem. Soc. Rev. 2018, 47, 533–557. [Google Scholar] [CrossRef]
- Sahadevan, S.A.; Abhervé, A.; Monni, N.; Sáenz de Pipaón, C.; Galán-Mascarós, J.R.; Waerenborgh, J.C.; Vieira, B.J.C.; Auban-Senzier, P.; Pillet, S.; Bendeif, E.-E.; et al. Conducting Anilate-Based Mixed-Valence Fe(II)Fe(III) Coordination Polymer: Small-Polaron Hopping Model for Oxalate-Type Fe(II)Fe(III) 2D Networks. J. Am. Chem. Soc. 2018, 140, 12611–12621. [Google Scholar] [CrossRef]
- Gómez-Claramunt, P.; Benmansour, S.; Hernández-Paredes, A.; Cerezo-Navarrete, C.; Rodríguez-Fernández, C.; Canet-Ferrer, J.; Cantarero, A.; Gómez-García, C.J. Tuning the Structure and Properties of Lanthanoid Coordination Polymers with an Asymmetric Anilato Ligand. Magnetochemistry 2018, 4, 6. [Google Scholar] [CrossRef]
- Mondal, A.; Roy, S.; Konar, S. Remarkable Energy Barrier for Magnetization Reversal in 3D and 2D Dysprosium-Chloranilate-Based Coordination Polymers. Chemistry 2020, 26, 8774–8783. [Google Scholar] [CrossRef] [PubMed]
- Kharitonov, A.D.; Trofimova, O.Y.; Meshcheryakova, I.N.; Fukin, G.K.; Khrizanforov, M.N.; Budnikova, Y.H.; Bogomyakov, A.S.; Aysin, R.R.; Kovalenko, K.A.; Piskunov, A.V. 2D-Metal-organic coordination polymers of lanthanides (La(III), Pr(III) and Nd(III)) with redox-active dioxolene bridging ligand. CrystEngComm 2020, 22, 4675–4679. [Google Scholar] [CrossRef]
- Calbo, J.; Golomb, M.J.; Walsh, A. Redox-active metal–organic frameworks for energy conversion and storage. J. Mater. Chem. A 2019, 7, 16571–16597. [Google Scholar] [CrossRef]
- Trofimova, O.Y.; Ershova, I.V.; Maleeva, A.V.; Cherkasov, A.V.; Khrizanforov, M.N.; Kovalenko, K.A.; Bogomyakov, A.S.; Piskunov, A.V. Synthesis and Properties of Manganese (II) and Nickel (II) 1-D Coordination Polymers Based on 2,5-di-hydroxy-3,6-di-tert-butyl-para-quinone. J. Inorg. Organomet. Polym. Mater. 2024, 34, 2779–2787. [Google Scholar] [CrossRef]
- Trofimova, O.Y.; Meshcheryakova, I.N.; Druzhkov, N.O.; Ershova, I.V.; Maleeva, A.V.; Cherkasov, A.V.; Yakushev, I.A.; Dorovatovskii, P.V.; Aysin, R.R.; Piskunov, A.V. Structural diversity of cadmium coordination polymers based on an extended anilate-type ligand. CrystEngComm 2024, 26, 3077–3087. [Google Scholar] [CrossRef]
- Collins, K.A.; Saballos, R.J.; Fataftah, M.S.; Puggioni, D.; Rondinelli, J.M.; Freedman, D.E. Synthetic investigation of competing magnetic interactions in 2D metal–chloranilate radical frameworks. Chem. Sci. 2020, 11, 5922–5928. [Google Scholar] [CrossRef]
- DeGayner, J.A.; Wang, K.; Harris, T.D. A Ferric Semiquinoid Single-Chain Magnet via Thermally-Switchable Metal–Ligand Electron Transfer. J. Am. Chem. Soc. 2018, 140, 6550–6553. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, L.; Li, J.; Lin, W.; Wang, Z. Switching on supramolecular catalysis via cavity mediation and electrostatic regulation. Angew. Chem. 2016, 128, 12970–12974. [Google Scholar] [CrossRef]
- Cullen, W.; Misuraca, M.C.; Hunter, C.A.; Williams, N.H.; Ward, M.D. Highly efficient catalysis of the Kemp elimination in the cavity of a cubic coordination cage. Nat. Chem. 2016, 8, 231–236. [Google Scholar] [CrossRef]
- Burke, B.P.; Grantham, W.; Burke, M.J.; Nichol, G.S.; Roberts, D.; Renard, I.; Hargreaves, R.; Cawthorne, C.; Archibald, S.J.; Lusby, P.J. Visualizing kinetically robust CoIII4L6 assemblies in Vivo: SPECT imaging of the encapsulated [99mTc] TcO4– anion. J. Am. Chem. Soc. 2018, 140, 16877–16881. [Google Scholar] [CrossRef]
- Holloway, L.R.; Bogie, P.M.; Lyon, Y.; Ngai, C.; Miller, T.F.; Julian, R.R.; Hooley, R.J. Tandem reactivity of a self-assembled cage catalyst with endohedral acid groups. J. Am. Chem. Soc. 2018, 140, 8078–8081. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Chu, D.; Gong, W.; Cui, Y.; Liu, Y. Metal-organic cages with missing linker defects. Angew. Chem. 2021, 133, 9181–9187. [Google Scholar] [CrossRef]
- Meshcheryakova, I.N.; Trofimova, O.Y.; Druzhkov, N.O.; Pashanova, K.I.; Yakushev, I.A.; Dorovatovskii, P.V.; Khrizanforov, M.N.; Budnikova, Y.G.; Aisin, R.R.; Piskunov, A.V. Magnesium and Nickel Complexes with Bis (p-iminoquinone) Redox-Active Ligand. Russ. J. Coord. Chem. 2021, 47, 307–318. [Google Scholar] [CrossRef]
- Liu, W.; Stoddart, J.F. Emergent behavior in nanoconfined molecular containers. Chem 2021, 7, 919–947. [Google Scholar] [CrossRef]
- Cherkasova, A.V.; Cherkasov, A.V.; Martyanov, K.A.; Bogomyakov, A.S.; Khrustalev, V.N.; Kissel, A.A.; Kozhanov, K.A.; Kuropatov, V.A.; Cherkasov, V.K. Self-assembly of a metal–organic cage-like structure bearing cofacial redox-active bis-(o-semiquinone) copper (II) units. Dalton Trans. 2023, 52, 15107–15114. [Google Scholar] [CrossRef]
- Cherkasova, A.V.; Kuropatov, V.A.; Romanenko, G.V.; Cherkasov, A.V.; Fukin, G.K.; Bogomyakov, A.S.; Martyanov, K.A.; Bubnov, M.P.; Cherkasov, V.K. Cobalt and nickel homobinuclear metallomacrocycles based on the redox-active bis-semiquinonato ligands as potential functional absorbing materials. Polyhedron 2024, 247, 116717–116723. [Google Scholar] [CrossRef]
- Chen, L.; Chen, Q.; Wu, M.; Jiang, F.; Hong, M. Controllable coordination-driven self-assembly: From discrete metallocages to infinite cage-based frameworks. Acc. Chem. Res. 2015, 48, 201–210. [Google Scholar] [CrossRef]
- Mercuri, M.L.; Congiu, F.; Concas, G.; Sahadevan, S.A. Recent Advances on Anilato-Based Molecular Materials with Magnetic and/or Conducting Properties. Magnetochemistry 2017, 3, 17. [Google Scholar] [CrossRef]
- Benmansour, S.; Abhervé, A.; Gómez-Claramunt, P.; Vallés-García, C.; Gomez-Garcia, C.J. Nano-sheets of 2D magnetic and conducting Fe(II)/Fe(III) mixed-valence MOFs. ACS Appl. Mater. Interfaces 2017, 9, 26210–26218. [Google Scholar] [CrossRef]
- Chen, J.; Sekine, Y.; Komatsumaru, Y.; Hayami, S.; Miyasaka, H. Thermally Induced Valence Tautomeric Transition in a Two-Dimensional Fe-Tetraoxolene Honeycomb Network. Angew. Chem. Int. Ed. 2018, 57, 12043–12047. [Google Scholar] [CrossRef]
- Kingsbury, C.J.; Abrahams, B.F.; Auckett, J.E.; Chevreau, H.; Dharma, A.D.; Duyker, S.; He, Q.; Hua, C.; Hudson, T.A.; Murray, K.S.; et al. Square Grid Metal–Chloranilate Networks as Robust HostSystems for GuestSorption. Chem. Eur. J. 2019, 25, 5222–5234. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Li, A.-C.; Popovs, I.; Kaveevivitchai, W.; Chen, J.-L.; Chou, K.-C.; Kuof, T.-S.; Chen, T.-H. Elucidating metal and ligand redox activities of a copper-benzoquinoid coordination polymer as the cathode for lithium-ion batteries. J. Mater. Chem. A 2019, 7, 23770–23774. [Google Scholar] [CrossRef]
- Martínez-Hernández, C.; Gómez-Claramunt, P.; Benmansour, S.; Gómez-García, C.J. Pre- and post-syntheticmodulation of the ordering temperatures in a family of anilato-based magnets. Dalton Trans. 2019, 48, 13212–13223. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; DeGayner, J.A.; Sun, L.; Zee, D.Z.; Harris, T.D. Reversible redox switching of magnetic order and electrical conductivity in a 2D manganese benzoquinoid framework. Chem. Sci. 2019, 10, 4652–4661. [Google Scholar] [CrossRef]
- Darago, L.E.; Aubrey, M.L.; Yu, C.J.; Gonzalez, M.I.; Long, J.R. Electronic Conductivity, Ferrimagnetic Ordering, and Reductive Insertion Mediated by Organic Mixed-Valence in a Ferric Semiquinoid Metal–Organic Framework. J. Am. Chem. Soc. 2015, 137, 15703–15711. [Google Scholar] [CrossRef]
- Jeon, I.-R.; Negru, B.; Van Duyne, R.P.; Harris, T.D. A 2D Semiquinone Radical-Containing Microporous Magnet with Solvent-Induced Switching from Tc = 26 to 80 K. J. Am. Chem. Soc. 2015, 137, 15699–15702. [Google Scholar] [CrossRef]
- Oggianu, M.; Bertolotti, F.; Manna, F.; Congiu, F.; Cappai, A.; Melis, C.; Concas, G.; Avarvari, N.; Masciocchi, N.; Mercuri, M.L. Slow magnetic relaxation in a heteroleptic anilate-based Dy III metal–organic framework. Dalton Trans. 2024, 53, 14265–14271. [Google Scholar] [CrossRef]
- Monni, N.; Dey, S.; García-López, V.; Oggianu, M.; Baldoví, J.J.; Mercuri, M.L.; Clemente-León, M.; Coronado, E. Tunable SIM properties in a family of 3D anilato-based lanthanide-MOFs. Inorg. Chem. Front. 2024, 11, 5913–5923. [Google Scholar] [CrossRef]
- Hua, C. Structure directing effects of the tetraphenylphosphonium cation upon the formation of lanthanoid anilate coordination polymers. Eur. J. Inorg. Chem. 2024, 27, e202400026. [Google Scholar] [CrossRef]
- Piskunov, A.V.; Meshcheryakova, I.N.; Fukin, G.K.; Smolyaninov, I.V.; Khamaletdinova, N.M.; Kuznetsova, O.V. Tin (IV) complexes based on 2-hydroxy-3,6-di-tert-butyl-para-benzoquinone: Syntheses, structures, and electrochemical behavior in solution. Russ. J. Coord. Chem. 2014, 40, 205–215. [Google Scholar] [CrossRef]
- Khamaletdinova, N.M.; Meshcheryakova, I.N.; Piskunov, A.V.; Kuznetsova, O.V. Experimental and theoretical study of the vibrational spectra of tin(IV) complexes based on 2-hydroxy-3,6-di-tert-butyl-para-benzoquianone. J. Struct. Chem. 2015, 56, 233–242. [Google Scholar] [CrossRef]
- Piskunov, A.V.; Meshcheryakova, I.N.; Fukin, G.K.; Smolyaninov, I.V.; Berberova, N.T. Syntheses, structures, and electrochemical properties of the tin (IV) complexes based on the 2-hydroxy-4-N-(phenyl)-3,6-di-tert-butyl-p-iminobenzoquinone ligand. Russ. J. Coord. Chem. 2017, 43, 816–827. [Google Scholar] [CrossRef]
- Meshcheryakova, I.N.; Shavyrin, A.S.; Trofimova, O.Y.; Cherkasov, A.V.; Fukin, G.K.; Piskunov, A.V. Octacoordinated tin (IV) complexes bearing oxy-p-benzoquinone and oxy-p-iminobenzoquinone ligands: Structural investigations and dynamics of coordination sphere in solution. J. Mol. Struct. 2020, 1220, 128734–128741. [Google Scholar] [CrossRef]
- Batsanov, S.S. The atomic radii of the elements. Russ. J. Inorg. Chem. 1991, 36, 1694–1705. [Google Scholar]
- Perrin, D.D.; Armarego, W.L.F. Purification of Laboratory Chemicals, 4th ed.; Butterworth-Heinemann: Oxford, UK, 1980. [Google Scholar]
- Abakumov, G.A.; Cherkasov, V.K.; Kocherova, T.N.; Druzhkov, N.O.; Kurskii, Y.A.; Abakumova, L.G. Quinonimines and aminoquinones, the reaction products of 3,6-di (tert-butyl)-o-benzoquinone with primary and secondary amines. Russ. Chem. Bull. 2006, 55, 1195–1199. [Google Scholar] [CrossRef]
- Svetogorov, R.D.; Dorovatovskii, P.V.; Lazarenko, V.A. Belok/XSA Diffraction Beamline for Studying Crystalline Samples at Kurchatov Synchrotron Radiation Source. Cryst. Res. Technol. 2020, 55, 1900184. [Google Scholar] [CrossRef]
- Kabsch, W. XDS. Acta Crystallogr. Sect. D 2010, 66, 125–132. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. A-Struct. Chem. 2015, 71, 3. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- The Cambridge Crystallographic Data Centre. Available online: http://www.ccdc.cam.ac.uk/data_request/cif (accessed on 23 September 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meshcheryakova, I.N.; Druzhkov, N.O.; Yakushev, I.A.; Arsenyeva, K.V.; Klimashevskaya, A.V.; Piskunov, A.V. Bis [4,4′-(1,3-Phenylenebis(azanylylidene))-bis(3,6-di-tert-butyl-2-oxycyclohexa-2,5-dien-1-one)-bis(dimethylsulfoxide)nickel(II)]. Molbank 2024, 2024, M1890. https://doi.org/10.3390/M1890
Meshcheryakova IN, Druzhkov NO, Yakushev IA, Arsenyeva KV, Klimashevskaya AV, Piskunov AV. Bis [4,4′-(1,3-Phenylenebis(azanylylidene))-bis(3,6-di-tert-butyl-2-oxycyclohexa-2,5-dien-1-one)-bis(dimethylsulfoxide)nickel(II)]. Molbank. 2024; 2024(4):M1890. https://doi.org/10.3390/M1890
Chicago/Turabian StyleMeshcheryakova, Irina N., Nikolay O. Druzhkov, Ilya A. Yakushev, Kseniya V. Arsenyeva, Anastasiya V. Klimashevskaya, and Alexandr V. Piskunov. 2024. "Bis [4,4′-(1,3-Phenylenebis(azanylylidene))-bis(3,6-di-tert-butyl-2-oxycyclohexa-2,5-dien-1-one)-bis(dimethylsulfoxide)nickel(II)]" Molbank 2024, no. 4: M1890. https://doi.org/10.3390/M1890
APA StyleMeshcheryakova, I. N., Druzhkov, N. O., Yakushev, I. A., Arsenyeva, K. V., Klimashevskaya, A. V., & Piskunov, A. V. (2024). Bis [4,4′-(1,3-Phenylenebis(azanylylidene))-bis(3,6-di-tert-butyl-2-oxycyclohexa-2,5-dien-1-one)-bis(dimethylsulfoxide)nickel(II)]. Molbank, 2024(4), M1890. https://doi.org/10.3390/M1890





