(R)-3,3,6-Trimethyl-3,3a,4,5-tetrahydro-1H-cyclopenta[c]furan
Abstract
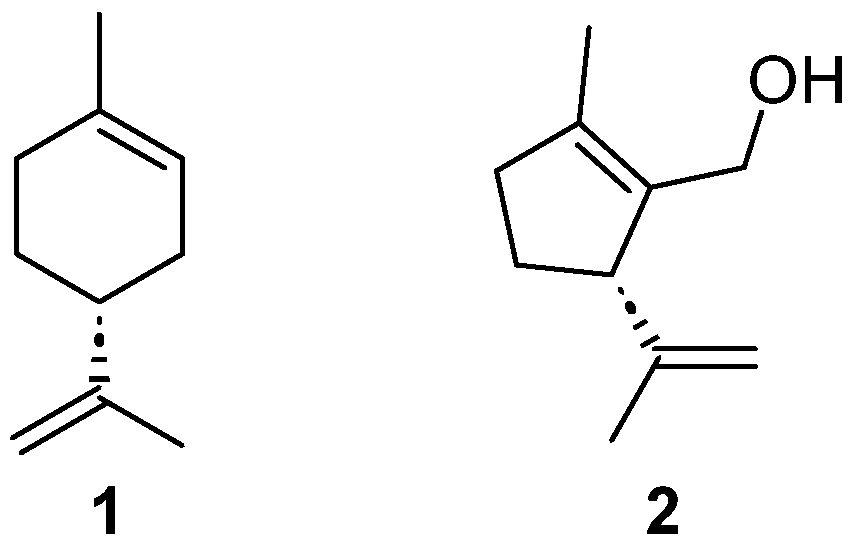
1. Introduction
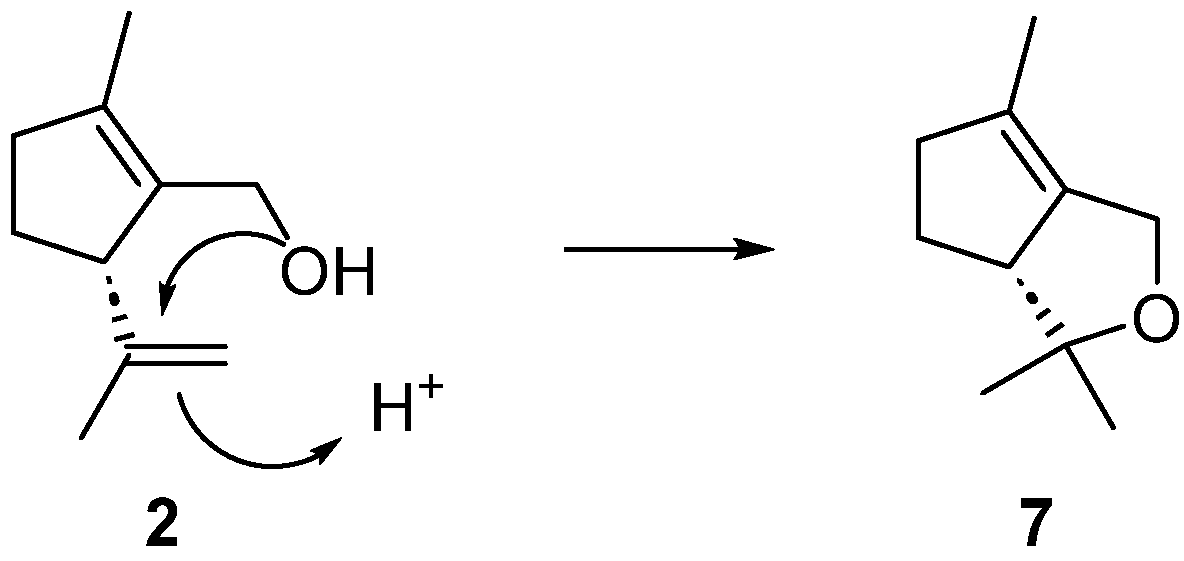
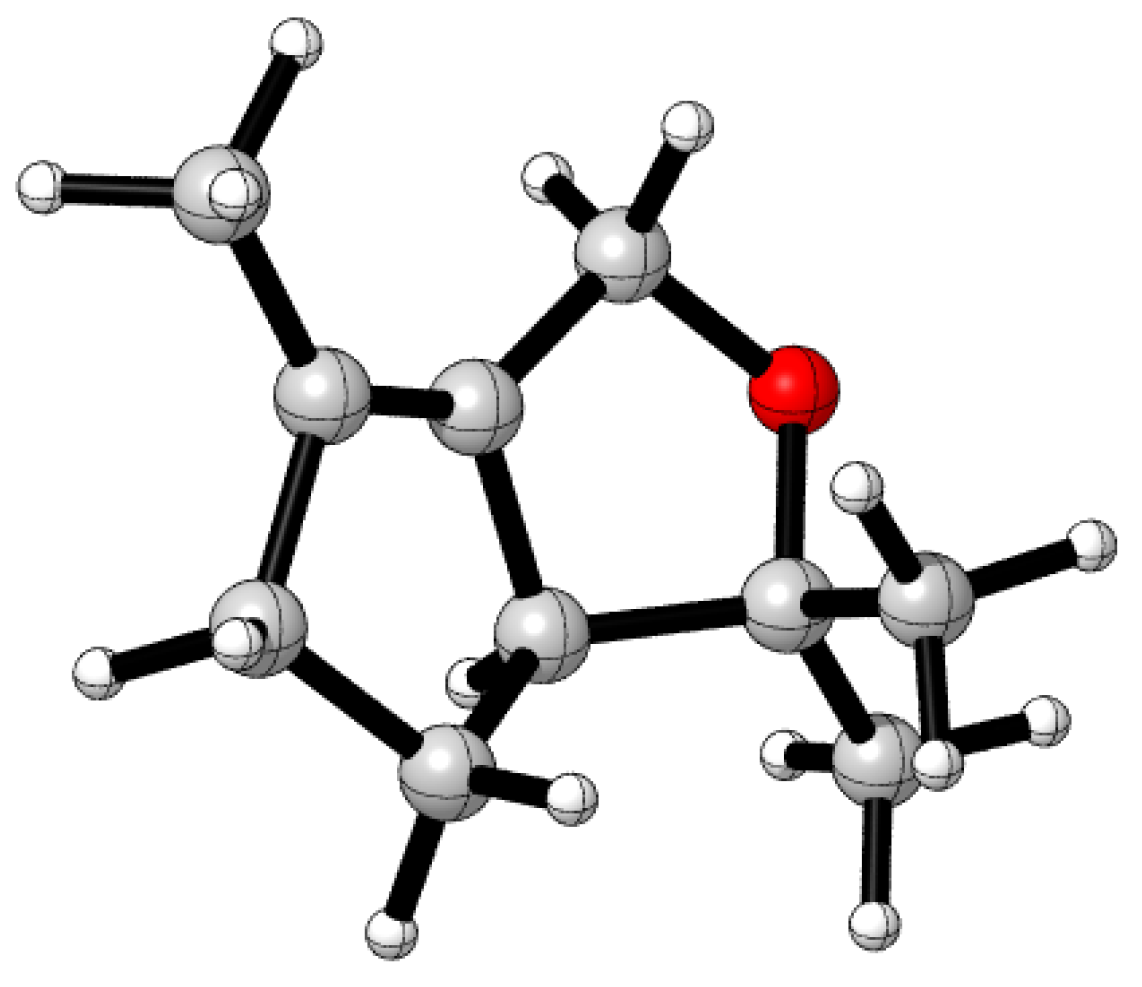
2. Results and Discussion
3. Experimental
3.1. General Experimental
3.2. (R)-3,3,6-Trimethyl-3,3a,4,5-tetrahydro-1H-cyclopenta[c]furan (7)
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wolinsky, J.; Nelson, D. The Synthesis of (+)-Matatabiether and Related Methylcyclopentane monoterpenes. Tetrahedron 1969, 25, 3767–3774. [Google Scholar] [CrossRef]
- Takeshita, H.; Hatsui, T.; Kato, N.; Masuda, T.; Tagoshi, H. Functionalized cyclopentane derivatives from the photoadducts of methyl 2,4-dioxopentanoate olefins: Alternative synthesis of dl-dihydroiridodial and dl-chrysomelidial. Chem. Lett. 1982, 8, 1153–1156. [Google Scholar] [CrossRef]
- Kato, N.; Nakanishi, K.; Takeshita, H. Synthetic photochemistry. XXXIV. Synthetic strategy of 5-8-5-membered tricyclic higher terpenoids based on the condensation of two optically-active iridoids, C-synthons obtained from a photocycloadduct of methyl 2,4-dioxopentanoate-isoprene, and its application to a synthesis of the basic carbon skeleton of fusicoccane. Bull. Chem. Soc. Jpn. 1986, 59, 1109–1123. [Google Scholar] [CrossRef]
- Lee, E.; Park, T.-K.; Tae, Y.; Cho, S.-D.; Chung, J.-S. Synthesis of hydroazulenic intermediates. Bull. Korean Chem. Soc. 1987, 8, 127–128. [Google Scholar] [CrossRef]
- Kato, N.; Kamitamari, M.; Naganuma, S.; Arita, H.; Takeshita, H. Stereoselective epoxidation of 1-iridene derivatives. Total syntheses of methyl chokolate A and Matatabiether. Heterocycles 1990, 30, 341–345. [Google Scholar]
- Naemura, K.; Hasegawa, T.; Miyabe, H.; Chikamatsu, H. The biogenetic-type cyclization of the unsaturated monocyclic alcohol with formic acid; facile synthesis of the tricarbocyclic alcohol, (+)-2-epi-allo-cedrol. Bull. Chem. Soc. Jpn. 1992, 65, 203–209. [Google Scholar] [CrossRef]
- Okamoto, H.; Arita, H.; Kato, N.; Takeshita, H. Total synthesis of (−)-cotylenol, a fungal metabolite having a leaf growth activity. Chem. Lett. 1994, 12, 2335–2338. [Google Scholar] [CrossRef]
- Srikrishna, A.; Babu, N.C. An enantiospecific formal total synthesis of (−)-aplysin and (−)-debromoaplysin. Tetrahedron Lett. 2001, 42, 4913–4914. [Google Scholar] [CrossRef]
- Srikrishna, A.; Dethe, D.H. Enantiospecific First Total Synthesis and Assignment of Absolute Configuration of the Sesquiterpene (−)-Cucumin H. Org. Lett. 2003, 5, 2295–2298. [Google Scholar] [CrossRef]
- Srikrishna, A.; Babu, N.C.; Rao, M.S. A stereoselective total synthesis of (+)-α-herbertenola. Tetrahedron 2004, 60, 2125–2130. [Google Scholar] [CrossRef]
- Srikrishna, A.; Pardeshi, V.H. Enantiospecific total synthesis of aciphyllene. Tetrahedron 2010, 66, 8160–8168. [Google Scholar] [CrossRef]
- Srikrishna, A.; Sheth, V.M.; Nagaraju, G. Rhodium carbenoid mediated C-H activation of a tertiary methyl group: An enantiospecific approach to the angular triquinanes norsilphiperfolane and norcameroonanes. Synlett 2011, 16, 2343–2346. [Google Scholar] [CrossRef][Green Version]
- Zimmermann, N.; Hilgraf, R.; Lehmann, L.; Ibarra, D.; Francke, W. Stereoselective synthesis of trans-fused iridoid lactones and their identification in the parasitoid wasp Alloxysta victrix, part I: Dihydronepetalactones. Beilstein J. Org. Chem. 2012, 8, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Kuwata, K.; Hanaya, K.; Higashibayashi, S.; Sugai, T.; Shoji, M. Synthesis of the 1,2-seco fusicoccane diterpene skeleton by Stille coupling reaction between the highly functionalized A and C ring segments of cotylenin A. Tetrahedron 2017, 73, 6039–6045. [Google Scholar] [CrossRef]
- Evanno, L.; Belotti, D.; Toromanoff, E.; Cossy, J. Synthesis of 12-epi-Protopanaxadiol and Formal Synthesis of Ginsenoside Chikusetsusaponin-LT8. Eur. J. Org. Chem. 2019, 2019, 5970–5973. [Google Scholar] [CrossRef]
- Dethe, D.-H.; Nirpal, A.-K. Enantiospecific Total Synthesis of (−)-Japonicol C. Org. Lett. 2021, 23, 2648–2653. [Google Scholar] [CrossRef]
- Roberts, R.A.; Schuell, V.; Paquette, L.A. Electrophile-initiated ring-opening reactions of 2-methylene-6,6-dimethylbicyclo[3.1.0]hexanes. New methodology for the synthesis of highly functionalized 1,2,3-trisubstituted cyclopentenes. J. Org. Chem. 1983, 48, 2076–2084. [Google Scholar] [CrossRef]
- Wolinsky, J.; Barker, W. The Synthesis of 1-acetyl-4-Isopropenyl-1-Cyclopentene. J. Am. Chem. Soc. 1960, 82, 636. [Google Scholar] [CrossRef]
- Wolinsky, J.; Slabaugh, M.R.; Gibson, T. Synthesis of 4-(2-Methyl-5-Isopropenyl-1-cyclopentene-1-yl)butan-2-one. A By-Product in the Synthesis of Pseudoionone. J. Org. Chem. 1964, 29, 3740–3742. [Google Scholar] [CrossRef]
- Chabardes, P.; Kuntz, E.; Varagnat, J. Use of oxo-metallic derivatives in isomerization. Reactions of unsaturated alcohols. Tetrahedron 1977, 33, 1775–1783. [Google Scholar] [CrossRef]
- Erman, M.B.; Aul’chenko, I.S.; Kheifits, L.A.; Dulova, V.G.; Novikov, J.N.; Vol’pin, M.E. The rearrangement of tertiary propargyl alcohols to α,β-unsaturated aldehydes in the presence of polymeric organosilyl vanadates. Tetrahedron Lett. 1976, 34, 2981–2984. [Google Scholar] [CrossRef]
- Yunkyung, J.; Do-Young, K.; Yunsil, C.; Jae-Sang, R. Intramolecular hydroalkoxylation in Brønsted acidic ionic liquids and its application to the synthesis of (±)-centrolobine. Org. Biomol. Chem. 2011, 9, 374–378. [Google Scholar] [CrossRef]
- Coulombel, L.; Duñach, E. Triflic acid-catalysed cyclisation of unsaturated alcohols. Green Chem. 2004, 6, 499–501. [Google Scholar] [CrossRef]
- Dzudza, A.; Marks, T.J. Efficient Intramolecular Hydroalkoxylation/Cyclization of Unactivated Alkenols Mediated by Lanthanide Triflate Ionic Liquids. Org. Lett. 2009, 11, 1523–1526. [Google Scholar] [CrossRef] [PubMed]
- Stoychev, G.L.; Auer, A.A.; Izsak, R.; Neese, F. Self-Consistent Field Calculation of Nuclear Magnetic Resonance Chemical Shielding Constants Using Gauge-Including Atomic Orbitals and Approximate Two-Electron Integrals. J. Chem. Theory Comput. 2018, 14, 619–637. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 5.0. WIRES Comput. Molec. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Legault, C.Y. CYLview, version 1.0b; Université de Sherbrooke: Sherbrooke, QC, USA, 2009. Available online: http://www.cylview.org (accessed on 5 October 2024).

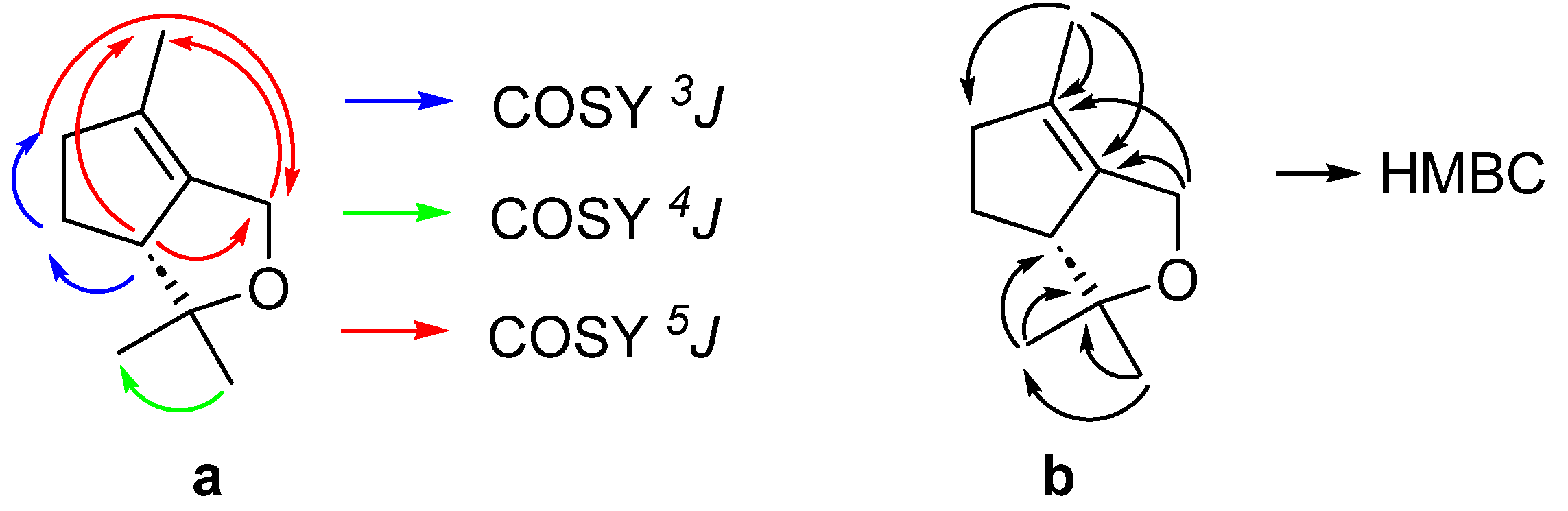
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marichal-Medina, D.M.; Rodríguez-Caro, J.F.; Afonso, M.M.; Palenzuela, J.A. (R)-3,3,6-Trimethyl-3,3a,4,5-tetrahydro-1H-cyclopenta[c]furan. Molbank 2024, 2024, M1902. https://doi.org/10.3390/M1902
Marichal-Medina DM, Rodríguez-Caro JF, Afonso MM, Palenzuela JA. (R)-3,3,6-Trimethyl-3,3a,4,5-tetrahydro-1H-cyclopenta[c]furan. Molbank. 2024; 2024(4):M1902. https://doi.org/10.3390/M1902
Chicago/Turabian StyleMarichal-Medina, Débora María, Juan Francisco Rodríguez-Caro, María M. Afonso, and José Antonio Palenzuela. 2024. "(R)-3,3,6-Trimethyl-3,3a,4,5-tetrahydro-1H-cyclopenta[c]furan" Molbank 2024, no. 4: M1902. https://doi.org/10.3390/M1902
APA StyleMarichal-Medina, D. M., Rodríguez-Caro, J. F., Afonso, M. M., & Palenzuela, J. A. (2024). (R)-3,3,6-Trimethyl-3,3a,4,5-tetrahydro-1H-cyclopenta[c]furan. Molbank, 2024(4), M1902. https://doi.org/10.3390/M1902





