2-([1,1′-Biphenyl]-4-yl)-5-[(E)-2-(3-methoxy-1-phenyl-1H-pyrazol-4-yl)ethenyl]-3,3-dimethyl-3H-indole
Abstract
:1. Introduction
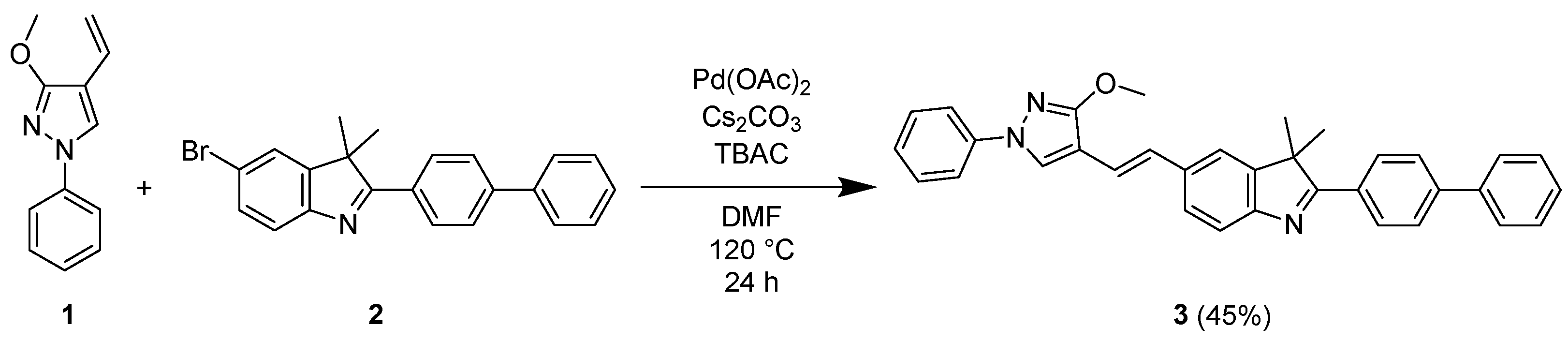
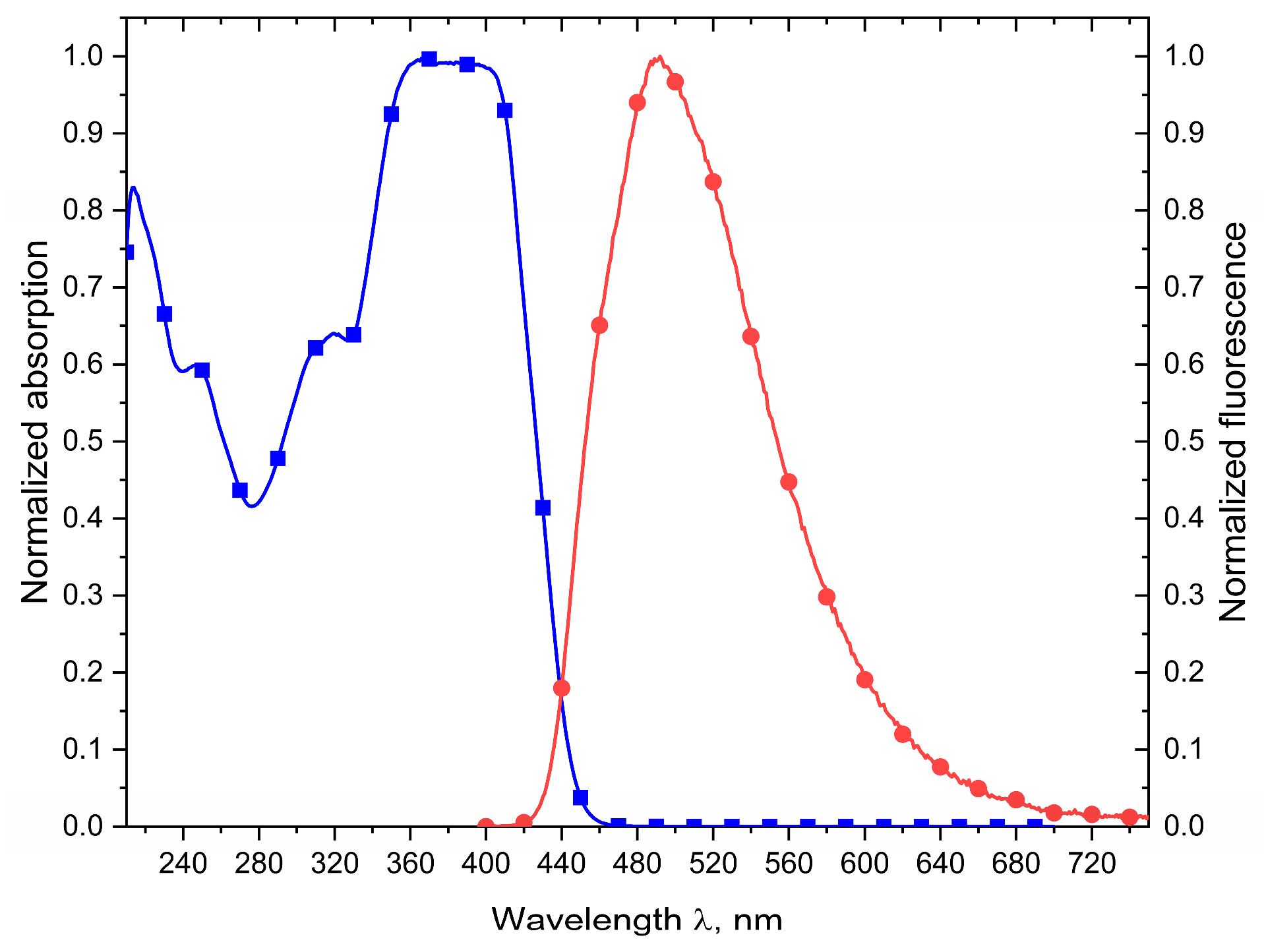
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tu, Z.; Lu, J.; Luo, X.; Hu, J.; Li, S.; Wang, Y.; Zheng, Y.; Zuo, J.; Pan, Y. Blue Axially Chiral Biphenyl Based Thermally Activated Delayed Fluorescence Materials for Efficient Circularly Polarized OLEDs. Adv. Opt. Mater. 2021, 9, 2100596. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Wu, X.; Chu, W.; Yin, S. Solution-Processable, High Current Efficiency Deep-Blue Organic Light-Emitting Diodes Based on Novel Biphenyl-Imidazole Derivatives. J. Mater. Chem. C 2020, 8, 11239–11251. [Google Scholar] [CrossRef]
- Xie, Y.; Han, L.; Ge, C.-S.; Cui, Y.-H.; Gao, J.-R. Novel Organic Dye Sensitizers Containing Fluorenyl and Biphenyl Moieties for Solar Cells. Chin. Chem. Lett. 2017, 28, 285–292. [Google Scholar] [CrossRef]
- Magomedov, A.; Sakai, N.; Kamarauskas, E.; Jokubauskaitė, G.; Franckevičius, M.; Jankauskas, V.; Snaith, H.J.; Getautis, V. Amorphous Hole-Transporting Material Based on 2,2′-Bis-substituted 1,1′-Biphenyl Scaffold for Application in Perovskite Solar Cells. Chem. Asian J. 2017, 12, 958–962. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Luo, Y.; Ye, J.; Liu, D.; Zhang, W. Rh-Catalyzed Enantioselective Desymmetric Hydrogenation of α-Acetamido-1,3-Indanediones Using Ether-Bridged Biphenyl Diphosphine Ligands. J. Am. Chem. Soc. 2023, 145, 21176–21182. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzani, R.; Kiriakidi, S.; Monari, M.; Lazzarini, I.; Bertuzzi, G.; López, C.S.; Bandini, M. Fluorinated Biphenyl Phosphine Ligands for Accelerated [Au(I)]-Catalysis. ACS Catal. 2024, 14, 6128–6136. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, T.; Li, B.; Xu, M.; Wang, Y. Design, Synthesis and Biological Evaluation of Biphenyl-Benzamides as Potent FtsZ Inhibitors. Eur. J. Med. Chem. 2022, 239, 114553. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Geetha, P.; Ramajayam, R. Isolation, Synthesis and Medicinal Chemistry of Biphenyl Analogs—A Review. Results Chem. 2023, 6, 101135. [Google Scholar] [CrossRef]
- Wang, T.; Cai, S.; Wang, M.; Zhang, W.; Zhang, K.; Chen, D.; Li, Z.; Jiang, S. Novel Biphenyl Pyridines as Potent Small-Molecule Inhibitors Targeting the Programmed Cell Death-1/Programmed Cell Death-Ligand 1 Interaction. J. Med. Chem. 2021, 64, 7390–7403. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Liao, C.; Li, Z.; Wang, Z.; Sun, Q.; Liu, C.; Yang, Y.; Tu, Z.; Jiang, S. Design, Synthesis, and Biological Evaluation of 1, 3-Disubstituted-Pyrazole Derivatives as New Class I and IIb Histone Deacetylase Inhibitors. Eur. J. Med. Chem. 2014, 86, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Asteian, A.; Blayo, A.-L.; He, Y.; Koenig, M.; Shin, Y.; Kuruvilla, D.S.; Corzo, C.A.; Cameron, M.D.; Lin, L.; Ruiz, C.; et al. Design, Synthesis, and Biological Evaluation of Indole Biphenylcarboxylic Acids as PPARγ Antagonists. ACS Med. Chem. Lett. 2015, 6, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Sawada, J.; Osawa, A.; Takeuchi, T.; Kaneda, M.; Oishi, S.; Fujii, N.; Asai, A.; Tanino, K.; Namba, K. Functional 1,3a,6a-Triazapentalene Scaffold: Design of Fluorescent Probes for Kinesin Spindle Protein (KSP). Bioorg. Med. Chem. Lett. 2016, 26, 5765–5769. [Google Scholar] [CrossRef] [PubMed]
- Denißen, M.; Nordmann, J.; Dziambor, J.; Mayer, B.; Frank, W.; Müller, T.J.J. Sequential Palladium Catalyzed Coupling–Cyclocondensation–Coupling (C3) Four-Component Synthesis of Intensively Blue Luminescent Biarylsubstituted Pyrazoles. RSC Adv. 2015, 5, 33838–33854. [Google Scholar] [CrossRef]
- Tomasulo, M.; Sortino, S.; Raymo, F.M. Bichromophoric Photochromes Based on the Opening and Closing of a Single Oxazine Ring. J. Org. Chem. 2008, 73, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Šachlevičiūtė, U.; Varvuolytė, G.; Bieliauskas, A.; Kleizienė, N.; Žukauskaitė, A.; Šačkus, A. 3,3,3′,3′-Tetramethyl-2,2′-Diphenyl-3H,3′H-5,5′-Biindole. Molbank 2020, 2020, M1146. [Google Scholar] [CrossRef]
- Arbačiauskienė, E.; Kazlauskas, K.; Miasojedovas, A.; Juršėnas, S.; Jankauskas, V.; Holzer, W.; Getautis, V.; Šačkus, A. Pyrazolyl-Substituted Polyconjugated Molecules for Optoelectronic Applications. Dyes Pigm. 2010, 85, 79–85. [Google Scholar] [CrossRef]
- Ali, H.A.; Ismail, M.A.; Fouda, A.E.-A.S.; Ghaith, E.A. A Fruitful Century for the Scalable Synthesis and Reactions of Biphenyl Derivatives: Applications and Biological Aspects. RSC Adv. 2023, 13, 18262–18305. [Google Scholar] [CrossRef] [PubMed]
- Cotugno, P.; Monopoli, A.; Ciminale, F.; Cioffi, N.; Nacci, A. Pd Nanoparticle Catalysed One-Pot Sequential Heck and Suzuki Couplings of Bromo-Chloroarenes in Ionic Liquids and Water. Org. Biomol. Chem. 2012, 10, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Varvuolytė, G.; Řezníčková, E.; Bieliauskas, A.; Kleizienė, N.; Vojáčková, V.; Opichalová, A.; Žukauskaitė, A.; Kryštof, V.; Šačkus, A. Synthesis and Photodynamic Activity of New 5-[(E)-2-(3-Alkoxy-1-phenyl-1H-pyrazol-4-yl)ethenyl]-2-phenyl-3H-indoles. Arch. Pharm. 2024, 357, e2400282. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varvuolytė, G.; Bieliauskas, A.; Kleizienė, N.; Žukauskaitė, A.; Šačkus, A. 2-([1,1′-Biphenyl]-4-yl)-5-[(E)-2-(3-methoxy-1-phenyl-1H-pyrazol-4-yl)ethenyl]-3,3-dimethyl-3H-indole. Molbank 2024, 2024, M1927. https://doi.org/10.3390/M1927
Varvuolytė G, Bieliauskas A, Kleizienė N, Žukauskaitė A, Šačkus A. 2-([1,1′-Biphenyl]-4-yl)-5-[(E)-2-(3-methoxy-1-phenyl-1H-pyrazol-4-yl)ethenyl]-3,3-dimethyl-3H-indole. Molbank. 2024; 2024(4):M1927. https://doi.org/10.3390/M1927
Chicago/Turabian StyleVarvuolytė, Gabrielė, Aurimas Bieliauskas, Neringa Kleizienė, Asta Žukauskaitė, and Algirdas Šačkus. 2024. "2-([1,1′-Biphenyl]-4-yl)-5-[(E)-2-(3-methoxy-1-phenyl-1H-pyrazol-4-yl)ethenyl]-3,3-dimethyl-3H-indole" Molbank 2024, no. 4: M1927. https://doi.org/10.3390/M1927
APA StyleVarvuolytė, G., Bieliauskas, A., Kleizienė, N., Žukauskaitė, A., & Šačkus, A. (2024). 2-([1,1′-Biphenyl]-4-yl)-5-[(E)-2-(3-methoxy-1-phenyl-1H-pyrazol-4-yl)ethenyl]-3,3-dimethyl-3H-indole. Molbank, 2024(4), M1927. https://doi.org/10.3390/M1927






