A New Synthetic Route for Preparation of 5-Methyl-5-Benzylhydantoin: X-Ray Analysis and Theoretical Calculations
Abstract
:1. Introduction
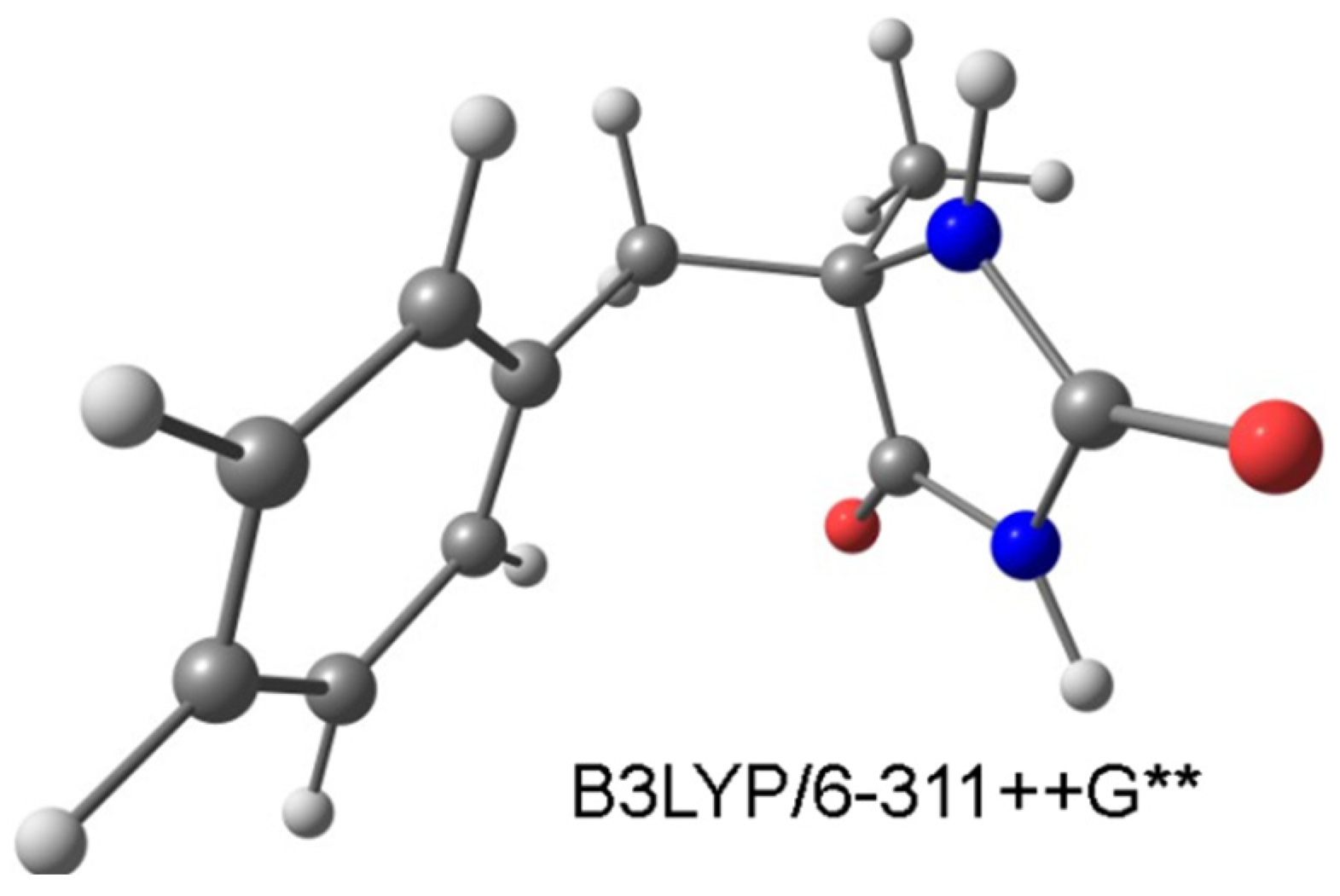
2. Results and Discussion
3. Materials and Methods
3.1. Preparation of 5-Methyl-5-Benzyl Hydantoin (Ours Modification)
3.2. Single-Crystal X-Ray Diffraction Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meusel, M.; Gütschow, M. Recent developments in hydantoin chemistry. A Review. Org. Prep. Proced. Int. 2004, 36, 391–443. [Google Scholar] [CrossRef]
- Konnert, L.; Lamaty, F.; Martinez, J.; Colacino, E. Recent advances in the synthesis of hydantoins: The state of the art of a valuable scaffold. Chem. Rev. 2017, 117, 13757–13809. [Google Scholar] [CrossRef] [PubMed]
- Wadghane, S.; Bhor, R.; Shinde, G.; Kolhe, M.; Pooja, R. A review on the some biological activities of the hydantoin derivatives. J. Drug Deliv. Ther. 2023, 13, 171–178. [Google Scholar] [CrossRef]
- Abida, A.M.; Asif, M. Study of some hyndantion derivatives as anticonvulsant agents. Prog. Chem. Biochem. Res. 2020, 3, 93–104. [Google Scholar] [CrossRef]
- Kochetkov, K.A.; Gorunova, O.N.; Bystrova, N. Biologically oriented hybrids of indole and hydantoin derivatives. Molecules 2023, 28, 602. [Google Scholar] [CrossRef]
- Hassanin, M.A.; Mustafa, M.; Hassan, H.A.; Beshr, E.A.M.; Elshennawy, A.T.M.; Aly, O.M. Hydantoin derivatives: A review on their anticancer activities. Octahedron Drug Res. 2024, 4, 61–74. [Google Scholar] [CrossRef]
- Cho, S.H.; Kim, S.H.; Shin, D. Recent applications of hydantoin and thiohydantoin in medicinal chemistry. Eur. J. Med. Chem. 2019, 164, 517–545. [Google Scholar] [CrossRef]
- Kumar, S.A.; Bhaskar, B.L. Spectroscopic and computational approach for the structure—Property relationship of hydantoin drug impurity: 1-methyl-5, 5-diphenylimidazolidine-2,4-dione. IOP Conf. Ser. Mater. Sci. Eng. 2019, 577, 012180. [Google Scholar] [CrossRef]
- Zhu, Q.F.; Pan, Y.H.; Xu, Z.X.; Li, R.M.; Qiu, G.G.; Xu, W.J.; Ke, X.B.; Wu, L.M.; Hu, X.M. Synthesis and potential anticonvulsant activity of new N-3-substituted 5,5-cyclopropanespirohydantoins. Eur. J. Med. Chem. 2009, 44, 296–302. [Google Scholar] [CrossRef]
- Byrtus, H.; Obniska, J.; Czopek, A.; Kaminski, K. Synthesis and anticonvulsant activity of new N-Mannich bases derived from 5-cyclopropyl-5-phenylhydantoins. Arch. Pharm. 2011, 344, 231–241. [Google Scholar] [CrossRef]
- Sondhi, S.M.; Singh, J.; Kumar, A.; Jamal, H.; Gupta, P.P. Synthesis of amidine and amide derivatives and their evaluation for anti-inflammatory and analgesic activities. Eur. J. Med. Chem. 2009, 44, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Guerra, A.S.H.; Do Nascimento Malta, D.J.; Morais Laranjeira, L.P.; Souza Maia, M.B.; Colaço, N.C.; de Lima, M.D.C.A.; Galdino, S.L.; Da Rocha Pitta, I.; Gonçalves-Silva, T. Anti-inflammatory and antinociceptive activities of indoleeimidazolidine derivatives. Int. Immunopharm. 2011, 11, 1816–1822. [Google Scholar] [CrossRef] [PubMed]
- Stilz, H.U.; Guba, W.; Jablonka, B.; Just, M.; Klingler, O.; Konig, W.; Wehner, V.; Zoller, G. Discovery of an orally active non-peptide fibrinogen receptor antagonist based on the hydantoin scaffold. J. Med. Chem. 2001, 44, 1158–1176. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.E.; Ouk, S.; Yoo, D.; Sawyers, C.L.; Chen, C.; Tran, C.; Wongvipat, J. Structure-activity relationship for thiohydantoin androgen receptor antagonists for castration-resistant prostate cancer (CRPC). J. Med. Chem. 2010, 53, 2779–2796. [Google Scholar] [CrossRef]
- Malancona, S.; Altamura, S.; Filocamo, G.; Kinzel, O.; Hernando, J.I.M.; Rowley, M.; Scarpelli, R.; Steinkühler, C.; Jones, P. Identification of MK-5710 ((8aS)-8-a-methyl-1,3-dioxo-2-[(1S,2R)-2-phenylcyclopropyl]-N-(1-phenyl-1H-pyrazol-5-yl)hexahydroimidazo [1,5-a]pyrazine-7(1H)-carboxamide), a potent smoothened antagonist for use in Hedgehog pathway dependent malignancies, Part 1. Bioorg. Med. Chem. Lett. 2011, 21, 4422–4428. [Google Scholar] [CrossRef]
- Kim, D.; Wang, L.; Caldwell, C.G.; Chen, P.; Finke, P.E.; Oates, B.; MacCoss, M.; Mills, S.G.; Malkowitz, L.; Gould, S.L.; et al. Design, synthesis, and SAR of heterocycle-containing antagonists of the human CCR5 receptor for the treatment of HIV-1 infection. Bioorg. Med. Chem. Lett. 2001, 11, 3103–3106. [Google Scholar] [CrossRef]
- Romine, J.L.; St. Laurent, D.R.; Leet, J.E.; Martin, S.W.; Serrano-Wu, M.H.; Yang, F.K.; Gao, M.; O’Boyle, D.R.; Lemm, J.A.; Sun, J.H.; et al. Inhibitors of HCV NS5A: From iminothiazolidinones to symmetrical stilbenes. ACS Med. Chem. Lett. 2011, 2, 224–229. [Google Scholar] [CrossRef]
- Trišović, N.; Timić, T.; Divljaković, J.; Rogan, J.; Poleti, D.; Savić, M.M.; Ušćumlić, G. Synthesis, structural and biological characterization of 5-phenylhydantoin derivatives as potential anticonvulsant agents. Monatsh. für Chem. 2012, 143, 1451–1457. [Google Scholar] [CrossRef]
- Hashmi, I.A.; Aslam, A.; Ali, S.K.; Ahmed, V.-U.; Ali, F.I. Synthesis of hydantoins, thiohydantoins, and glycocyamidines under solvent-free conditions. Synth. Commun. 2010, 40, 2869–2874. [Google Scholar] [CrossRef]
- Bucherer, H.T.; Fischbeck, H.T. Hexahydrodiphenylamine and its derivatives. J. Prakt. Chem. 1934, 140, 69–89. [Google Scholar]
- Bergs, Hydantoinderivate. Ger. Pat. 566094 1929. Chem. Abstr. 1933, 27, 1001. [Google Scholar]
- Bucherer, H.T.; Steiner, W.J. Über die reaktionen der α–oxy und α–aminonitrilen. Synthese von hydantoinen. Prakt. Chem. 1934, 140, 291. [Google Scholar]
- Bucherer, H.T. Über die bildung substituiereter hydantoine aus aldehyden und ketonen. Prakt. Chem. 1934, 141, 5. [Google Scholar] [CrossRef]
- Urech, F. Ueber lacturaminsäure und lactylharnstoff. Liebigs Ann. 1873, 165, 99–103. (In German) [Google Scholar] [CrossRef]
- Herbst, R.; Johnson, T. Researches on hydantoins. L.1 The synthesis of hydantoins possessing the properties of hypnotics. J. Amer. Chem. Soc. 1932, 54, 2463. [Google Scholar] [CrossRef]
- Cherneva, E.; Buyukliev, R.; Burdjiev, N.; Michailova, R.; Bakalova, A. Pd(II) and Pd(IV) complexes with new hydantoin based ligand. Synthesis, characterization, computational and pharmacological studies. Bulg. Chem. Commun. 2018, 50, 237–242. [Google Scholar]
- Farrugia, L.J. Wingx and Ortep for Windows: An Update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Chattopadhyay, B.; Mukherjee, A.K.; Narendra, N.; Hemantha, H.P.; Sureshbabu, V.; Helliwell, M.; Mukherjee, M. Supramolecular architectures in 5,5′-substituted hydantoins: Crystal structures and hirshfeld surface analyses. Cryst. Growth Des. 2010, 10, 4476–4484. [Google Scholar] [CrossRef]
- Lazić, A.; Radovanović, L.; Rogan, J.; Valentić, N.; Janjić, G.; Đorđević, I.; Trišović, N. Exploring the supramolecular profile of 5-phenylhydantoins. Cryst. Eng. Comm. 2023, 25, 3637–3654. [Google Scholar] [CrossRef]
- Giunchi, A.; Rivalta, A.; Bedoya-Martínez, N.; Schrode, B.; Braun, D.; Werzer, O.; Venuti, E.; Della Valle, R.G. Surface induced phenytoin polymorph. 1. Full structure solution by combining grazing incidence X-ray diffraction and crystal structure prediction. Cryst. Growth Des. 2019, 19, 6067–6073. [Google Scholar] [CrossRef]
- Brown, I.D. On the geometry of O–H⋯ O hydrogen bonds. Acta Crystallogr. Sect. A Found. Crystallogr. 1976, 32, 24–31. [Google Scholar] [CrossRef]
- Taylor, R.; Kennard, O.; Versichel, W. Geometry of the nitrogen-hydrogen…oxygen-carbon (NH…O:C) hydrogen bond. 2. Three-center (bifurcated) and four-center (trifurcated) bonds. J. Am. Chem. Soc. 1984, 106, 244–248. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio calculation of vibrational absorption and circular dichroism spectra using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Paar, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision C.02; Gaussian Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Daskalova, L.I.; Velcheva, E.A.; Binev, I.G. Changes in the IR spectra and structures of pyridine-3-carboxamides-d0 and -d2 caused by their conversion into azanions-d0 and -d1: Experimental and computational studies. J. Mol. Struct. 2007, 826, 198–204. [Google Scholar] [CrossRef]
- Yancheva, D.; Stoyanov, S.; Anichina, K.; Nikolova, S.; Velcheva, E.; Stamboliyska, B. Combined infrared spectroscopic and computational study on simpler capsaicin derivatives and their anion intermediates in the scavenging of free radicals. Chem. Phys. 2020, 535, 110763. [Google Scholar] [CrossRef]
- Tachikawa, H.A.; Kawabata, H. Structures and electronic states of permethyloligosilane radical ions with all-trans form Sin(CH3)2n+2(±) (n = 2–6): A Density Functional Theory study. J. Chem. Theory Comp. 2007, 3, 184–193. [Google Scholar] [CrossRef]
- Stoyanov, S.; Yancheva, D.; Stamboliyska, B. DFT study on IR spectral and structural changes caused by the conversion of substituted benzophenones into ketyl radicals. Comput. Theor. Chem. 2014, 1046, 57–63. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Rigaku, O. Crysalis PRO; Rigaku Oxford Diffraction: Yarnton, UK, 2015. [Google Scholar]
- Sheldrick, G.M. A short history of shelx. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. Olex2: A Complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]



| D—H···A | D—H, Å | H···A, Å | D···A, Å | D—H···A, ° |
|---|---|---|---|---|
| N32—H32···O41 ii | 1.02 (3) | 1.81 (3) | 2.831 (3) | 176 (3) |
| N31—H31···O42 iii | 0.91 (4) | 1.88 (4) | 2.781 (5) | 173 (3) |
| N12—H12···O21 | 0.91 (4) | 1.92 (5) | 2.789 (4) | 159 (4) |
| N11—H11···O22 iv | 0.92 (5) | 1.93 (5) | 2.835 (5) | 166 (4) |
| C61—H61A···O42 i | 0.96 (4) | 2.53 (5) | 3.409 (5) | 153 (3) |
| C61—H61B···O41 iv | 0.96 (4) | 2.67 (5) | 3.444 (5) | 137 (2) |
| Parameters | Exp. | Theor. |
|---|---|---|
| Bond lengths (Å) | ||
| C22-O22 | 1.21 | 1.21 |
| C22-N12 | 1.35 | 1.37 |
| C42-O42 | 1.20 | 1.21 |
| C42-N42 | 1.33 | 1.36 |
| C52-C62 | 1.51 | 1.53 |
| C52-C72 | 1.54 | 1.55 |
| Angles (°) | ||
| N12-C52-C62 | 111.7 | 111.8 |
| N12-C52-C72 | 111.4 | 113.2 |
| N32-C42-C52 | 107.7 | 106.4 |
| O22-C22-N32 | 128.6 | 128.9 |
| O42-C42-N32 | 125.7 | 127.2 |
| Dihedral angles (°) | ||
| C42-C52-C72-C82 | −53.4 | 58.4 |
| C62-C52-N12-C22 | −123.8 | −119.3 |
| N12-C52-C72-C82 | −56.3 | 53.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherneva, E.; Buyukliev, R.; Shivachev, B.; Rusew, R.; Bakalova, A. A New Synthetic Route for Preparation of 5-Methyl-5-Benzylhydantoin: X-Ray Analysis and Theoretical Calculations. Molbank 2025, 2025, M1956. https://doi.org/10.3390/M1956
Cherneva E, Buyukliev R, Shivachev B, Rusew R, Bakalova A. A New Synthetic Route for Preparation of 5-Methyl-5-Benzylhydantoin: X-Ray Analysis and Theoretical Calculations. Molbank. 2025; 2025(1):M1956. https://doi.org/10.3390/M1956
Chicago/Turabian StyleCherneva, Emiliya, Rossen Buyukliev, Boris Shivachev, Rusi Rusew, and Adriana Bakalova. 2025. "A New Synthetic Route for Preparation of 5-Methyl-5-Benzylhydantoin: X-Ray Analysis and Theoretical Calculations" Molbank 2025, no. 1: M1956. https://doi.org/10.3390/M1956
APA StyleCherneva, E., Buyukliev, R., Shivachev, B., Rusew, R., & Bakalova, A. (2025). A New Synthetic Route for Preparation of 5-Methyl-5-Benzylhydantoin: X-Ray Analysis and Theoretical Calculations. Molbank, 2025(1), M1956. https://doi.org/10.3390/M1956







