Effects of Nanowire Length and Surface Roughness on the Electrochemical Sensor Properties of Nafion-Free, Vertically Aligned Pt Nanowire Array Electrodes
Abstract
:1. Introduction
2. Experimental Section
2.1. Apparatus and Reagents
2.2. Synthesis and Fabrication of PtNWA

2.3. Electrochemical Measurements
3. Results and Discussion
3.1. Characterization of Pt Nanowires and Array Electrodes



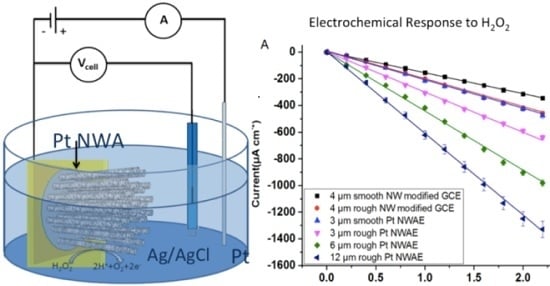
3.2. Electrochemical Response of the PtNWA towards H2O2


3.3. Amperometric Measurement



| Electrode | Eapp (mV) | Sensing Performance | Reference | ||
|---|---|---|---|---|---|
| LOD a (μM) | Sensitivity (μA·mM−1·cm−2) | LDR b (mM) | |||
| PtNP-CNT array | - | 1.5 | 140 | ~25 | [39] |
| Smooth Pt NW/GCE | −480 c | 47 | 130 | ~35 | [42] |
| Rough Pt NW/GCE | 20 | 170 | ~33 | ||
| PtNP/NAE | - | 1.0 | 194.6 | 0.020–20 | [44] |
| HRP/Pt nanowire | −100 c | - | 345 | ~2.5 | [45] |
| Marcroporous Au/nPts | 700c | 50 | 264 | ~0 | [46] |
| GC/RGO/PB/PTBO | 200 c | 1.5 | 420 | 0.005~0.6 | [47] |
| Pt NEA | 0 | 0.06 | 540 | 0.0001–60 | [38] |
| 12 μm rough PtNWA | −200 c | 2.85 | 654 | 0.01~4 | This work |
3.4. Selectivity Measurement

3.5. Detection of H2O2 in Real Water Samples

4. Conclusions
Author Contributions
Conflicts of Interest
References
- Halliwell, B.; Clement, M.V.; Long, L.H. Hydrogen peroxide in the human body. FEBS Lett. 2000, 486, 10–13. [Google Scholar] [CrossRef]
- Chen, J.; Rulkens, W.H.; Bruning, H. Photochemical elimination of phenols and cod in industrial wastewaters. Water Sci. Technol. 2007, 35, 231–238. [Google Scholar] [CrossRef]
- Rojas, M.R.; Leung, C.; Whitley, D.; Zhu, Y.; Arnold, R.G.; Sáez, A.E. Advanced oxidation of trace organics in water by hydrogen peroxide solar photolysis. Ind. Eng. Chem. Res. 2011, 50, 12479–12487. [Google Scholar] [CrossRef]
- Lu, C.-P.; Lin, C.-T.; Chang, C.-M.; Wu, S.-H.; Lo, L.-C. Nitrophenylboronic acids as highly chemoselective probes to detect hydrogen peroxide in foods and agricultural products. J. Agric. Food Chem. 2011, 59, 11403–11406. [Google Scholar] [CrossRef] [PubMed]
- Recio, E.; Álvarez-Rodríguez, M.L.; Rumbero, A.; Garzón, E.; Coque, J.J.R. Destruction of chloroanisoles by using a hydrogen peroxide activated method and its application to remove chloroanisoles from cork stoppers. J. Agric. Food Chem. 2011, 59, 12589–12597. [Google Scholar] [CrossRef] [PubMed]
- Anji Reddy, M.; Satya Kishore, M.; Pralong, V.; Caignaert, V.; Varadaraju, U.V.; Raveau, B. Electrochemical performance of VOMoO4 as negative electrode material for Li ion batteries. J. Power Sources 2007, 165, 509–512. [Google Scholar] [CrossRef]
- Raman, R.K.; Shukla, A.K. A direct borohydride/hydrogen peroxide fuel cell with reduced alkali crossover. Fuel Cells 2007, 7, 225–231. [Google Scholar] [CrossRef]
- Ikarashi, Y.; Tsuchiya, T.; Nakamura, A. Cytotoxicity of medical materials sterilized with vapour-phase hydrogen peroxide. Biomaterials 1995, 16, 177–183. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, Y. Improvement of peroxide bleaching yield and efficiency of TMP using glyoxal crosslink agents. Ind. Eng. Chem. Res. 2006, 45, 5813–5818. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, H.; Rui, Q.; Tian, Y. Detection of extracellular H2O2 released from human liver cancer cells based on TiO2 nanoneedles with enhanced electron transfer of cytochrome c. Anal. Chem. 2009, 81, 3035–3041. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Jiang, X.; Xie, Q.; Yao, S. A third-generation hydrogen peroxide biosensor based on horseradish peroxidase immobilized in a tetrathiafulvalene-tetracyanoquinodimethane/multiwalled carbon nanotubes film. Biosens. Bioelectron. 2008, 24, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Atwood, C.S.; Hartshorn, M.A.; Multhaup, G.; Goldstein, L.E.; Scarpa, R.C.; Cuajungco, M.P.; Gray, D.N.; Lim, J.; Moir, R.D.; et al. The Aβ peptide of Alzheimer’s disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry 1999, 38, 7609–7616. [Google Scholar] [CrossRef] [PubMed]
- López-Lázaro, M. Dual role of hydrogen peroxide in cancer: Possible relevance to cancer chemoprevention and therapy. Cancer Lett. 2007, 252, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Campanella, L.; Roversi, R.; Sammartino, M.P.; Tomassetti, M. Hydrogen peroxide determination in pharmaceutical formulations and cosmetics using a new catalase biosensor. J. Pharm. Biomed. Anal. 1998, 18, 105–116. [Google Scholar] [CrossRef]
- Niethammer, P.; Grabher, C.; Look, A.T.; Mitchison, T.J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 2009, 459, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, B. Hydroxyl radical and its scavengers in health and disease. Oxidative Med. Cell. Longev. 2011, 2011, 809696. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.H.; Wang, K.; Xia, X.H. Highly ordered platinum-nanotubule arrays for amperometric glucose sensing. Adv. Funct. Mater. 2005, 15, 803–809. [Google Scholar] [CrossRef]
- Park, S.; Chung, T.D.; Kim, H.C. Nonenzymatic glucose detection using mesoporous platinum. Anal. Chem. 2003, 75, 3046–3049. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.; Antczak, A.; Krol, M.; Pietras, T.; Shariati, B.; Bialasiewicz, P.; Jeczkowski, K.; Kula, P. Increased content of hydrogen peroxide in the exhaled breath of cigarette smokers. Eur. Respir. J. 1996, 9, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Long, L.H.; Evans, P.J.; Halliwell, B. Hydrogen peroxide in human urine: Implications for antioxidant defense and redox regulation. Biochem. Biophys. Res. Commun. 1999, 262, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Kolata, J.; Winterbourn, C.; Kramer, A.; Turner, R.; Weltmann, K.D.; Bröker, B.; Masur, K. Hydrogen peroxide: A central player in physical plasma-induced oxidative stress in human blood cells. Free Radic. Res. 2014, 48, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Hochbaum, A.I.; Chen, R.; Delgado, R.D.; Liang, W.; Garnett, E.C.; Najarian, M.; Majumdar, A.; Yang, P. Enhanced thermoelectric performance of rough silicon nanowires. Nature 2008, 451, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, D.; Bourgeois, L.; Wang, H.; Webley, P.A. Direct Electrodeposition of Porous Gold Nanowire Arrays for Biosensing Applications. Chem. Phys. Chem. 2009, 10, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.P.; Matisons, J.G. Synthesis of High Aspect-Ratio Gold Nanowires with Highly Porous Morphology. ISRN Nanomater. 2012, 502960. [Google Scholar] [CrossRef]
- Liu, Z.; Searson, P.C. Single Nanoporous Gold Nanowire Sensors. J. Phys. Chem. B 2006, 110, 4318–4322. [Google Scholar] [CrossRef] [PubMed]
- Benson, J.; Boukhalfa, S.; Magasinski, A.; Kvit, A.; Yushin, G. Chemical vapor deposition of aluminum annowires on metal substrates for electrical energy storage applications. ACS Nano 2012, 6, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, S.N. Analysis of the vapor-liquid-solid mechanism for nanowire growth and a model for this mechanism. Nano Lett. 2008, 8, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Xie, Y.; Wu, C.; Yang, J.; Li, Z.; Xu, F. Formation of silver nanowries through a sandwiched reduction process. Adv. Mater. 2003, 15, 405–408. [Google Scholar] [CrossRef]
- De Leo, M.; Pereira, F.C.; Moretto, L.M.; Scopece, P.; Polizzi, S.; Ugo, P. Towards a better understanding of gold electroless deposition in track-etched templates. Chem. Mater. 2007, 19, 5955–5964. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Cherevko, S.; Chung, C. Electroplating of metal nanotubes and nanowires in a high aspect-ratio nanotemplate. Electrochem. Commun. 2008, 10, 514–518. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, L.; Xu, W.; Zhang, J. Electrochemical Detection of Low Concentration Chloropropanol Using Silver Nanowire Array Electrodes in Aqueous Media. J. Nanosci. Nanotechnol. 2011, 11, 2283–2286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, W.; Da, J.; Wang, H.; Zhao, D.; Webley, P.A. Porous platinum nanowire arrays for direct ethanol fuel cell applications. Chem. Commun. 2009, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Bo, X.; Ndamanisha, J.C.; Bai, J.; Guo, L. Nonenzymatic amperometric sensor of hydrogen peroxide and glucose based on Pt nanoparticles/ordered mesoporous carbon nanocomposite. Talanta 2010, 82, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yuan, R.; Chai, Y.; Hu, F. Electrochemical sensing of hydrogen peroxide using metal nanoparticles: A review. Microchim. Acta 2013, 180, 15–32. [Google Scholar] [CrossRef]
- Gui, J.; Lu, Q.; Chen, Z.; Li, Y. Electrochemical Determination of Hydrogen Peroxide Using Gold–Platinum Bimetallic Nanoparticles Self-Assembled on l-Cysteamine Modified Glassy Carbon Electrode. Nanosci. Nanotechnol. Lett. 2013, 5, 637–642. [Google Scholar] [CrossRef]
- Zhong, F.; Zong, R.; Zhu, Y. Platinum Nanowire Array Electrochemical Sensor: Fabrication and Characterization. J. Nanosci. Nanotechnol. 2009, 9, 2437–2441. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Gao, F.; Gu, Z.; Pien, M.; Sun, H. A novel 3-D fabrication of platinum nanoparticles decorated microcarbon pillars electrode for high sensitivity detection of hydrogen peroxide. Sens. Actuators B Chem. 2013, 181, 57–64. [Google Scholar] [CrossRef]
- Yang, M.; Qu, F.; Lu, Y.; He, Y.; Shen, G.; Yu, R. Platinum nanowire nanoelectrode for the fabrication of biosensors. Biomaterials 2006, 27, 5944–5950. [Google Scholar] [CrossRef] [PubMed]
- Hrapvoic, S.; Liu, Y.; Male, K.B.; Luong, J. Electrochemical biosensing platforms using platinum nanoparticles and carbon nanotubes. Anal. Chem. 2004, 76, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Tong, D.H.; Tran, P.D.; Pham, X.T.T.; Le, T.T.T.; Dang, M.C.; van Rijn, C.J. The nanofabrication of Pt nanowire arrays at the wafer-scale and its application in glucose detection. Adv. Nat. Sci. Nanosci. Nanotechnol. 2010, 1, 015011. [Google Scholar] [CrossRef]
- Ruan, D.; Gao, F.; Gu, Z. Current controlled growth mechanism of surface roughed Pt nanowires and their electrochemical property. J. Electrochem. Soc. 2014, 161, D666–D671. [Google Scholar] [CrossRef]
- Gao, F.; Li, Z.; Ruan, D.; Gu, Z. Synthesis of surface roughed Pt nanowires and their application as electrochemical sensors for hydrogen peroxide detection. J. Nanosci. Nanotechnol. 2014, 14, 6599–6605. [Google Scholar] [CrossRef]
- Gao, F.; Liu, Z.; Zhou, G.; Yang, J.C.; Gu, Z. Fast diffusion and void formation in a two-segment copper-tin lead-free nanowire system with one-dimensional confinement. Sci. Adv. Mater. 2012, 4, 881–887. [Google Scholar] [CrossRef]
- Jamal, M.; Xu, J.; Razeeb, K.M. Disposable biosensor based on immobilisation of glutamate oxidase on Pt/Au nanowire array electrode. Biosens. Bioelectron. 2010, 26, 1420–1424. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Hasan, M.; Mischieri, J.; Mathewson, A.; Razeeb, K.M. Fabrication of horseradish peroxidase modified 3D Pt nanowire array electrode and its electro-catalytical activity towards H2O2. ECS Trans. 2011, 35, 53–59. [Google Scholar]
- Lee, Y.J.; Park, J.Y.; Kim, Y.; Ko, J.W. Amperometric sensing of hydrogen perox-ide via highly roughened macroporous gold/platinum nanoparticles electrode. Curr. Appl. Phys. 2011, 11, 211–216. [Google Scholar] [CrossRef]
- Bai, X.; Chen, G.; Shiu, K.K. Electrochemical biosensor based on reduced graphene oxide modified electrode with Prussian blue and poly(toluidine blue O) coating. Electrochim. Acta 2013, 89, 454–460. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Leung, C.; Gao, F.; Gu, Z. Effects of Nanowire Length and Surface Roughness on the Electrochemical Sensor Properties of Nafion-Free, Vertically Aligned Pt Nanowire Array Electrodes. Sensors 2015, 15, 22473-22489. https://doi.org/10.3390/s150922473
Li Z, Leung C, Gao F, Gu Z. Effects of Nanowire Length and Surface Roughness on the Electrochemical Sensor Properties of Nafion-Free, Vertically Aligned Pt Nanowire Array Electrodes. Sensors. 2015; 15(9):22473-22489. https://doi.org/10.3390/s150922473
Chicago/Turabian StyleLi, Zhiyang, Calvin Leung, Fan Gao, and Zhiyong Gu. 2015. "Effects of Nanowire Length and Surface Roughness on the Electrochemical Sensor Properties of Nafion-Free, Vertically Aligned Pt Nanowire Array Electrodes" Sensors 15, no. 9: 22473-22489. https://doi.org/10.3390/s150922473