Characterization of a Time-Resolved Diffuse Optical Spectroscopy Prototype Using Low-Cost, Compact Single Photon Avalanche Detectors for Tissue Optics Applications
Abstract
:1. Introduction
2. TR-DOS Prototype
2.1. Light Sources
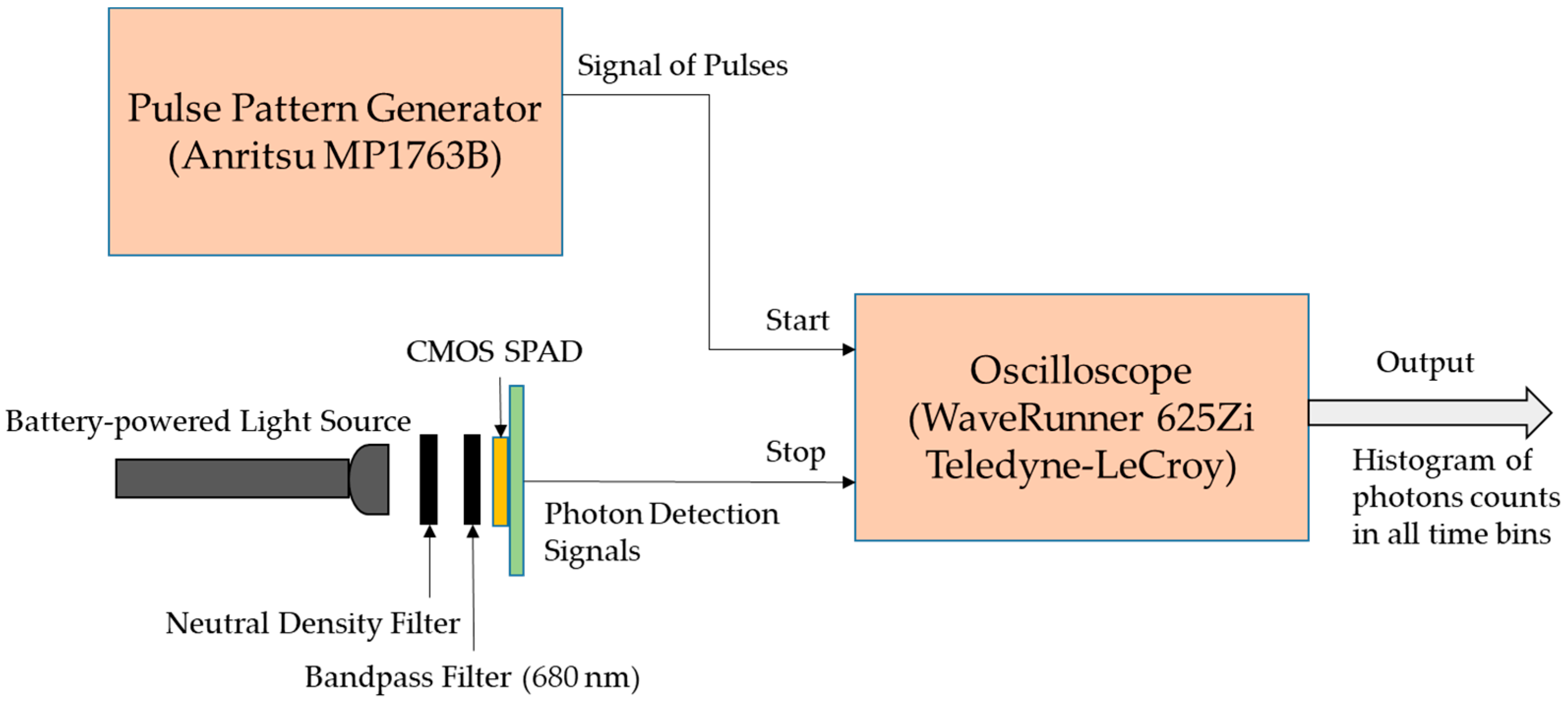
2.2. Photon Counting and Timing Subsystem
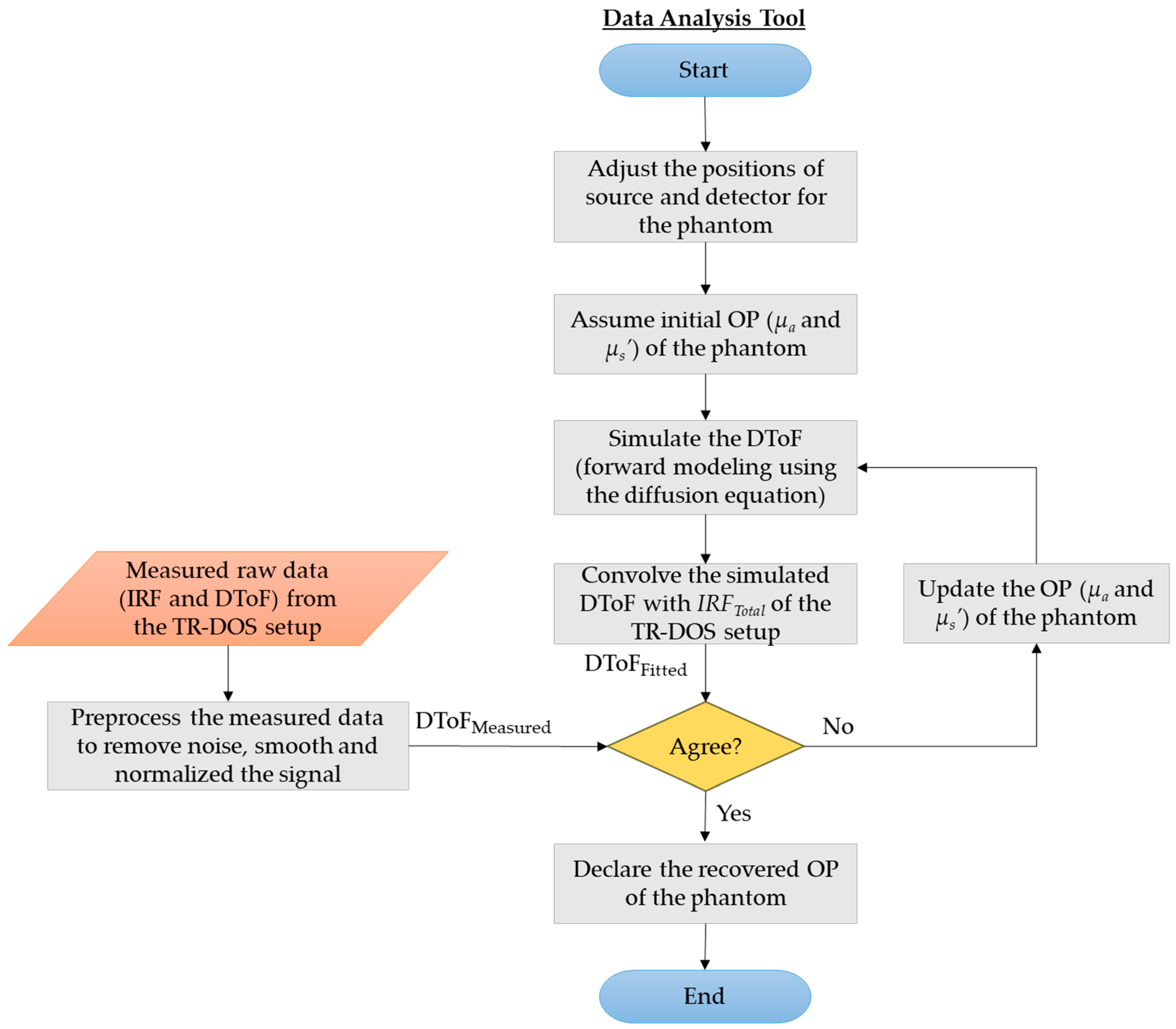
2.3. Data Analysis Tool
3. Characterization Methods
3.1. Basic Instrumental Performance Protocol
3.1.1. Light Power
3.1.2. Differential non-linearity (DNL) of Photon Timing
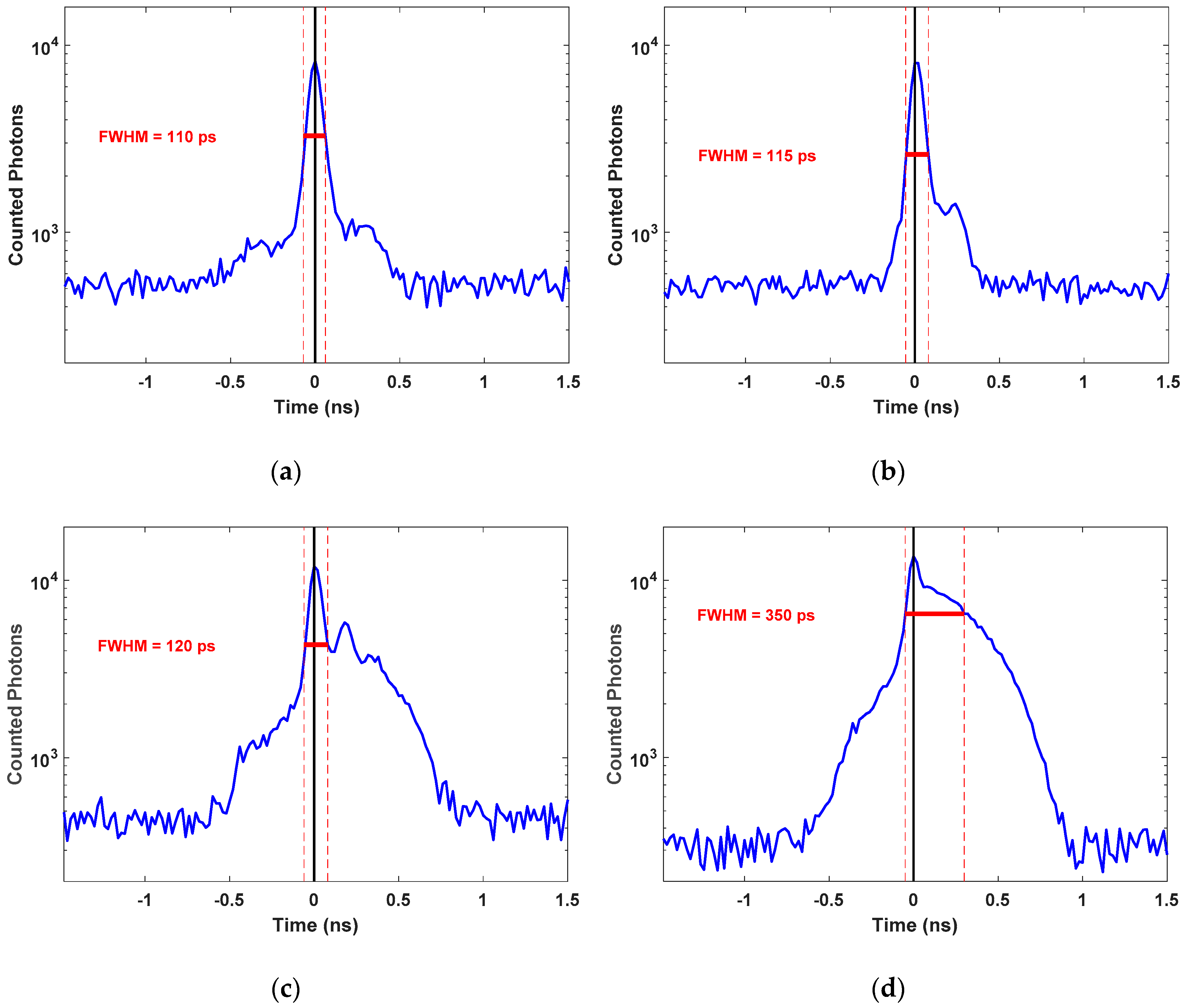
3.1.3. Total IRF of the TR-DOS Setup
3.2. Optical Properties Quantification of Homogeneous Phantoms
3.2.1. Accuracy Assessment
3.2.2. Linearity Assessment
3.2.3. Preparation of Phantoms
3.2.4. Data Acquisition and Preprocessing
4. Results and Discussions
4.1. Differential Non-Linearity
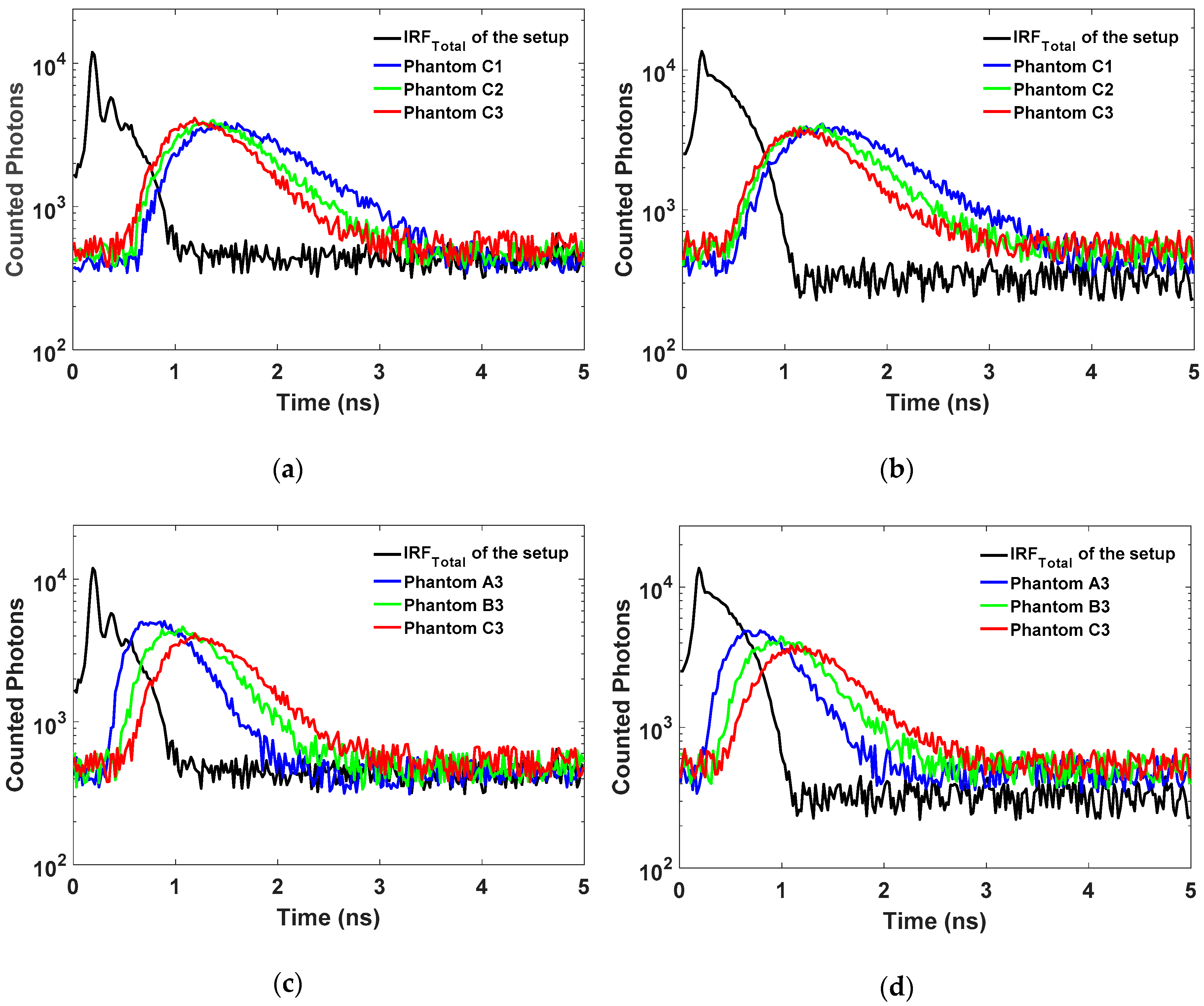
4.2. Total Instrument Response Function (IRFTotal)
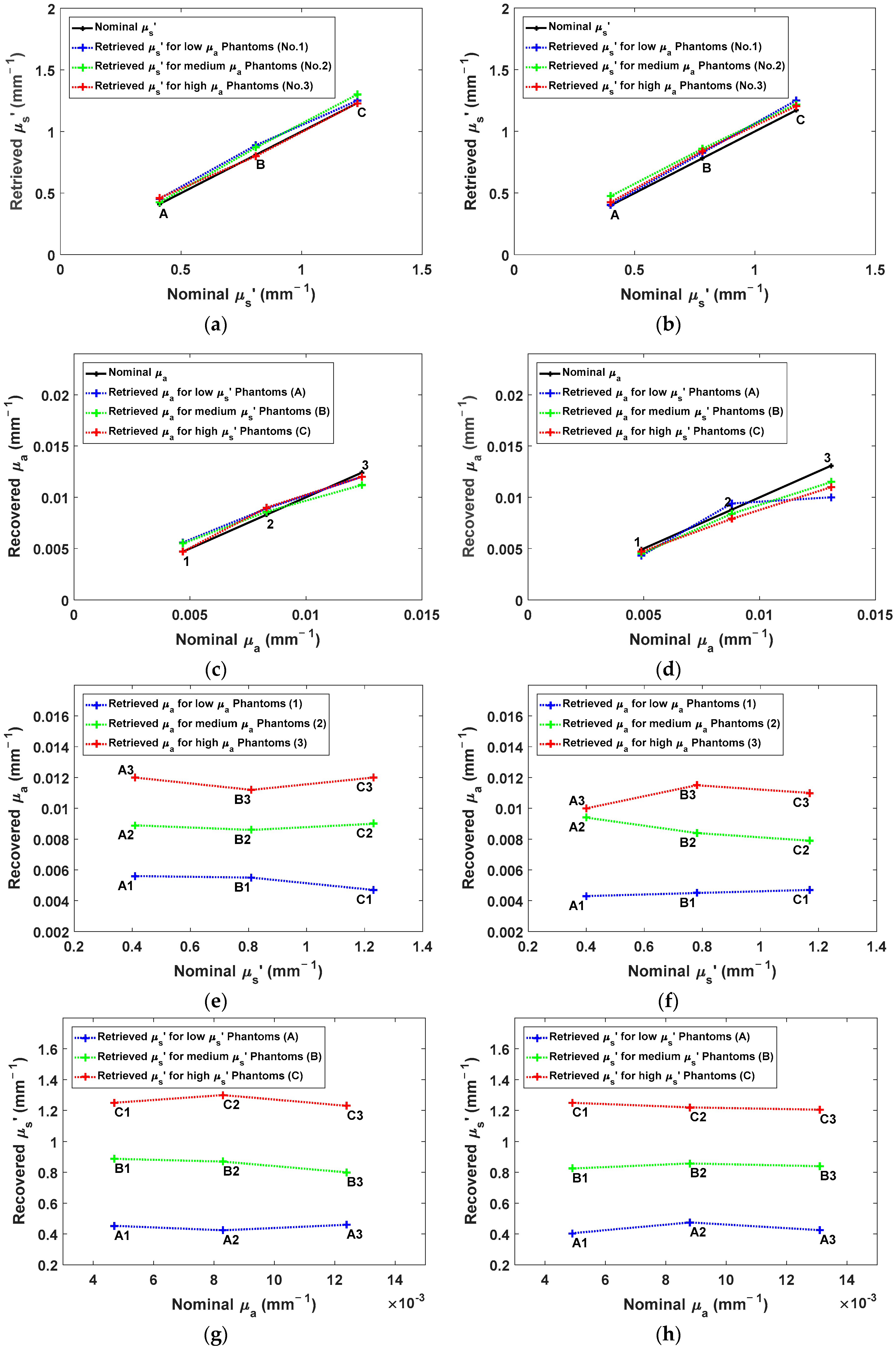
4.3. The Accuracy of the OP Quantification
4.4. The Linearity of the OP Quantification
4.5. Evaluation of this Prototype and the Potential Applications
4.6. Potential Developments of SPAD Detectors for Tissue Optics Applications
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alayed, M.; Deen, M. Time-resolved diffuse optical spectroscopy and imaging using solid-state detectors: Characteristics, present status, and research challenges. Sensors 2017, 17, 2115. [Google Scholar] [CrossRef] [PubMed]
- Pifferi, A.; Contini, D.; Dalla Mora, A.; Farina, A.; Spinelli, L.; Torricelli, A. New frontiers in time-domain diffuse optics, a review. J. Biomed. Opt. 2016, 21, 091310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Application of Near Infrared Spectroscopy in Biomedicine; Jue, T.; Masuda, K. (Eds.) Springer: Boston, MA, USA, 2013; ISBN 978-1-4614-6252-1. [Google Scholar]
- Yamada, Y.; Okawa, S. Diffuse optical tomography: Present status and its future. Opt. Rev. 2014, 21, 185–205. [Google Scholar] [CrossRef]
- Durduran, T.; Choe, R.; Baker, W.B.; Yodh, A.G. Diffuse optics for tissue monitoring and tomography. Rep. Prog. Phys. 2010, 73, 076701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martelli, F.; Del Bianco, S.; Ismaelli, A.; Zaccanti, G. Light Propagation through Biological Tissue and Other Diffusive Media: Theory, Solutions, and Software; SPIE Press: Bellingham, WA USA, 2009. [Google Scholar]
- Patterson, M.S.; Chance, B.; Wilson, B.C. Time resolved reflectance and transmittance for the noninvasive measurement of tissue optical properties. Appl. Opt. 1989, 28, 2331–23360. [Google Scholar] [CrossRef] [PubMed]
- Arridge, S.R.; Schotland, J.C. Optical tomography: Forward and inverse problems. Inverse Prob. 2009, 25, 123010. [Google Scholar] [CrossRef]
- Martelli, F.; Del Bianco, S.; Spinelli, L.; Cavalieri, S.; Di Ninni, P.; Binzoni, T.; Jelzow, A.; Macdonald, R.; Wabnitz, H. Optimal estimation reconstruction of the optical properties of a two-layered tissue phantom from time-resolved single-distance measurements. J. Biomed. Opt. 2015, 20, 115001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alayed, M.; Naser, M.; Aden-Ali, I.; Deen, M. Time-resolved diffuse optical tomography system using an accelerated inverse problem solver. Opt. Express 2018, 26, 963–979. [Google Scholar] [CrossRef] [PubMed]
- Hillman, E. Optical brain imaging in vivo: Techniques and applications from animal to man. J. Biomed. Opt. 2007, 12, 051402. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Quaresima, V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage 2012, 63, 921–935. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, A.; Contini, D.; Pifferi, A.; Caffini, M.; Re, R.; Zucchelli, L.; Spinelli, L. Time domain functional NIRS imaging for human brain mapping. Neuroimage 2014, 85, 28–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, F.; Zhao, H.; Yamada, Y. Improvement of image quality in diffuse optical tomography by use of full time-resolved data. Appl. Opt. 2002, 41, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Dalla Mora, A.; Contini, D.; Arridge, S.; Martelli, F.; Tosi, A.; Boso, G.; Farina, A.; Durduran, T.; Martinenghi, E.; Torricelli, A.; et al. Towards next-generation time-domain diffuse optics for extreme depth penetration and sensitivity. Biomed. Opt. Express 2015, 6, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Mora, A.; Martinenghi, E.; Contini, D.; Tosi, A.; Boso, G.; Durduran, T.; Arridge, S.; Martelli, F.; Farina, A.; Torricelli, A.; et al. Fast silicon photomultiplier improves signal harvesting and reduces complexity in time-domain diffuse optics. Opt. Express 2015, 23, 13937–13946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naser, M.; Deen, M. Time-domain diffuse optical tomography using recursive direct method of calculating Jacobian at selected temporal points. Biomed. Phys. Eng. Express 2015, 1, 045207. [Google Scholar] [CrossRef]
- Buttafava, M.; Martinenghi, E.; Tamborini, D.; Contini, D.; Dalla Mora, A.; Renna, M.; Torricelli, A.; Pifferi, A.; Zappa, F.; Tosi, A. A compact two-wavelength time-domain NIRS system based on SiPM and pulsed diode lasers. IEEE Photonics J. 2017, 9, 1–14. [Google Scholar] [CrossRef]
- Deen, M.; Basu, P. Silicon Photonics: Fundamentals and Devices; John Wiley & Sons: Chichester, UK, 2012; Volume 44. [Google Scholar]
- Bruschini, C.; Homulle, H.; Charbon, E. Ten years of biophotonics single-photon SPAD imager applications: Retrospective and outlook. In Proc. SPIE 10069, Multiphoton Microscopy in the Biomedical Sciences XVII; SPIE: Bellingham, WA, USA, 2017; Volume 10069. [Google Scholar]
- Pavia, J.; Wolf, M.; Charbon, E. Single-photon avalanche diode imagers applied to near-infrared imaging. IEEE J. Sel. Top. Quantum Electron. 2014, 20, 291–298. [Google Scholar] [CrossRef]
- Pavia, J.; Scandini, M.; Lindner, S.; Wolf, M.; Charbon, E. A 1 × 400 backside-illuminated SPAD sensor with 49.7 ps resolution, 30 pJ/sample TDCs fabricated in 3D CMOS technology for near-infrared optical tomography. IEEE J. Solid-State Circuits 2015, 50, 2406–2418. [Google Scholar] [CrossRef]
- Ghioni, M.; Gulinatti, A.; Rech, I.; Zappa, F.; Cova, S. Progress in silicon single-photon avalanche diodes. IEEE J. Sel. Top. Quantum Electron. 2007, 13, 852–862. [Google Scholar] [CrossRef]
- Lee, M.; Ximenes, A.; Padmanabhan, P.; Wang, T.; Huang, K.; Yamashita, Y.; Yaung, D.; Charbon, E. High-Performance Back-Illuminated Three-Dimensional Stacked Single-Photon Avalanche Diode Implemented in 45-nm CMOS Technology. IEEE J. Sel. Top. Quantum Electron. 2018, 24, 1–9. [Google Scholar] [CrossRef]
- Palubiak, D.; Li, Z.; Deen, M. Afterpulsing characteristics of free-running and time-gated single-photon avalanche diodes in 130-nm CMOS. IEEE Trans. Electron. Devices 2015, 62, 3727–3733. [Google Scholar] [CrossRef]
- Cheng, Z.; Palubiak, D.; Zheng, X.; Deen, M.; Peng, H. Impact of silicide layer on single photon avalanche diodes in a 130 nm CMOS process. J. Phys. D: Appl. Phys. 2016, 49, 345105. [Google Scholar] [CrossRef]
- Palubiak, D.; El-Desouki, M.; Marinov, O.; Deen, M.; Fang, Q. High-speed, single-photon avalanche-photodiode imager for biomedical applications. IEEE Sens. J. 2011, 11, 2401–2412. [Google Scholar] [CrossRef]
- PicoQuant. Picosecond Pulsed Sources. Available online: https://www.picoquant.com/products/category/picosecond-pulsed-sources/ldh-series-picosecond-pulsed-diode-laser-heads (accessed on 17 April 2018).
- PicoQuant. Picosecond Pulsed Sources—Fiber Coupling. Available online: https://www.picoquant.com/products/category/picosecond-pulsed-sources/ldh-series-picosecond-pulsed-diode-laser-heads#custom2 (accessed on 17 April 2018).
- MP1763B Pulse Pattern Generator Operation Manual. Available online: https://www.anritsu.com/en-us/test-measurement/support/downloads/manuals/dwl008621 (accessed on 19 April 2018).
- PicoQuant. Picosecond Pulsed Drivers—PDL 800-B. Available online: https://www.picoquant.com/products/category/picosecond-pulsed-driver/pdl-800-b-picosecond-pulsed-diode-laser-driver (accessed on 18 April 2018).
- Teledyne LeCroy. WaveRunner 625Zi. Available online: http://teledynelecroy.com/oscilloscope/waverunner-6zi-oscilloscopes/waverunner-625zi (accessed on 18 April 2018).
- Fang, Q.; Boas, D. Monte Carlo simulation of photon migration in 3D turbid media accelerated by graphics processing units. Opt. Express 2009, 17, 20178–20190. [Google Scholar] [CrossRef] [PubMed]
- Liemert, A.; Kienle, A. Exact and efficient solution of the radiative transport equation for the semi-infinite medium. Sci. Rep. 2013, 3, 2018. [Google Scholar] [CrossRef] [PubMed]
- Pifferi, A.; Taroni, P.; Valentini, G.; Andersson-Engels, S. Real-time method for fitting time-resolved reflectance and transmittance measurements with a Monte Carlo model. Appl. Opt. 1998, 37, 2774–2780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wabnitz, H.; Taubert, D.; Mazurenka, M.; Steinkellner, O.; Jelzow, A.; Macdonald, R.; Milej, D.; Sawosz, P.; Kacprzak, M.; Liebert, A.; et al. Performance assessment of time-domain optical brain imagers, part 1: Basic instrumental performance protocol. J. Biomed. Opt. 2014, 19, 086010. [Google Scholar] [CrossRef] [PubMed]
- Pifferi, A.; Torricelli, A.; Bassi, A.; Taroni, P.; Cubeddu, R.; Wabnitz, H.; Grosenick, D.; Möller, M.; Macdonald, R.; Swartling, J.; et al. Performance assessment of photon migration instruments: The MEDPHOT protocol. Appl. Opt. 2005, 44, 2104–2114. [Google Scholar] [CrossRef] [PubMed]
- Newport Corporation. Available online: https://www.newport.com/ (accessed on 16 May 2018).
- 1830-C Optical Power Meter—Instruction Manual. Available online: https://www.equipland.com/objects/catalog/product/extras/1030_1830-c.pdf (accessed on 16 May 2018).
- International Electrotechnical Commission. Safety of Laser Products Part 1: Equipment Classification and Requirements; IEC Standard: Geneva, Switzerland, 2014; Volume IEC 60825-1:2014. [Google Scholar]
- Bérubé-Lauzière, Y.; Crotti, M.; Boucher, S.; Ettehadi, S.; Pichette, J.; Rech, I. Prospects on time-domain diffuse optical tomography based on time-correlated single photon counting for small animal imaging. J. Spectro 2016. [Google Scholar] [CrossRef]
- Liebert, A.; Wabnitz, H.; Grosenick, D.; Macdonald, R. Fiber dispersion in time domain measurements compromising the accuracy of determination of optical properties of strongly scattering media. J. Biomed. Opt. 2003, 8, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Milej, D.; Gerega, A.; Kacprzak, M.; Sawosz, P.; Weigl, W.; Maniewski, R.; Liebert, A. Time-resolved multi-channel optical system for assessment of brain oxygenation and perfusion by monitoring of diffuse reflectance and fluorescence. Opto-Electron. Rev. 2014, 22, 55–67. [Google Scholar] [CrossRef] [Green Version]
- Spinelli, L.; Martelli, F.; Farina, A.; Pifferi, A.; Torricelli, A.; Cubeddu, R.; Zaccanti, G. Calibration of scattering and absorption properties of a liquid diffusive medium at NIR wavelengths. Time-resolved method. Opt. Express 2007, 15, 6589–6604. [Google Scholar] [CrossRef] [PubMed]
- Ntziachristos, V.; Chance, B. Accuracy limits in the determination of absolute optical properties using time-resolved NIR spectroscopy. Med. Phys. 2001, 28, 1115–1124. [Google Scholar] [CrossRef]
- Cheong, W.F.; Prahl, S.A.; Welch, A.J. A review of the optical properties of biological. IEEE J. Quantum Electron. 1990, 26, 2166–2185. [Google Scholar] [CrossRef]
- Pogue, B.; Patterson, M. Review of tissue simulating phantoms for optical spectroscopy, imaging and dosimetry. J. Biomed. Opt. 2006, 11, 041102. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, L.; Botwicz, M.; Zolek, N.; Kacprzak, M.; Milej, D.; Sawosz, P.; Liebert, A.; Weigel, U.; Durduran, T.; Foschum, F.; et al. Determination of reference values for optical properties of liquid phantoms based on Intralipid and India ink. Biomed. Opt. Express 2014, 5, 2037–2053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contini, D.; Torricelli, A.; Pifferi, A.; Spinelli, L.; Paglia, F.; Cubeddu, R. Multi-channel time-resolved system for functional near infrared spectroscopy. Opt. Express 2006, 14, 5418–5432. [Google Scholar] [CrossRef] [PubMed]
- Pifferi, A.; Torricelli, A.; Taroni, P.; Comelli, D.; Bassi, A.; Cubeddu, R. Fully automated time domain spectrometer for the absorption and scattering characterization of diffusive media. Rev. Sci. Instrum. 2007, 78, 053103. [Google Scholar] [CrossRef]
- Re, R.; Contini, D.; Caffini, M.; Cubeddu, R.; Spinelli, L.; Torricelli, A. A compact time-resolved system for near infrared spectroscopy based on wavelength space multiplexing. Rev. Sci. Instrum. 2010, 81, 113101. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Martinenghi, E.; Dalla Mora, A.; Contini, D.; Pifferi, A.; Torricelli, A. Probe-hosted silicon photomultipliers for time-domain functional near-infrared spectroscopy: Phantom and in vivo tests. Neurophotonics 2016, 3, 045004. [Google Scholar] [CrossRef] [PubMed]
- Tyndall, D.; Rae, B.; Li, D.; Arlt, J.; Johnston, A.; Richardson, J.; Henderson, R. A High-Throughput Time-Resolved Mini-Silicon Photomultiplier with Embedded Fluorescence Lifetime Estimation in 0.13 μm CMOS. IEEE Trans. Biomed. Circuits Syst. 2012, 6, 562–570. [Google Scholar] [CrossRef] [PubMed]




| Phantom | 685 nm | 830 nm | n | ||
|---|---|---|---|---|---|
| μs’ (mm−1) | μa (mm−1) | μs’ (mm−1) | μa (mm−1) | ||
| A1 | 0.41 | 0.0047 | 0.4 | 0.0049 | 1.5 |
| A2 | 0.41 | 0.0083 | 0.4 | 0.0088 | 1.5 |
| A3 | 0.41 | 0.0124 | 0.4 | 0.0131 | 1.5 |
| B1 | 0.81 | 0.0047 | 0.78 | 0.0049 | 1.5 |
| B2 | 0.81 | 0.0083 | 0.78 | 0.0088 | 1.5 |
| B3 | 0.81 | 0.0124 | 0.78 | 0.0131 | 1.5 |
| C1 | 1.23 | 0.0047 | 1.17 | 0.0049 | 1.5 |
| C2 | 1.23 | 0.0083 | 1.17 | 0.0088 | 1.5 |
| C3 | 1.23 | 0.0124 | 1.17 | 0.0131 | 1.5 |
| % Errors in the Estimate of μs’ | % Errors in the Estimate of μa | |||||
|---|---|---|---|---|---|---|
| 685 nm Results | μs’ | |||||
| μa | A (0.41) | B (0.81) | C (1.23) | A (0.41) | B (0.81) | C (1.23) |
| 1 (0.0047) | 10.5 | 9.5 | 1.6 | 19 | 17 | 0 |
| 2 (0.0083) | 3.5 | 7.5 | 5.7 | 7.2 | 3.6 | 8.4 |
| 3 (0.0124) | 12 | −1.2 | 0 | −3.2 | −9.7 | −3.2 |
| 830 nm Results | μs’ | |||||
| μa | A (0.4) | B (0.78) | C (1.17) | A (0.4) | B (0.78) | C (1.17) |
| 1 (0.0049) | 1.3 | 5.8 | 6.8 | −12.2 | −8.2 | −4 |
| 2 (0.0088) | 18.8 | 10 | 4.3 | 6.8 | −4.5 | −10.2 |
| 3 (0.0131) | 6.3 | 7.7 | 3 | −23.7 | −12.2 | −16 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alayed, M.; Palubiak, D.P.; Deen, M.J. Characterization of a Time-Resolved Diffuse Optical Spectroscopy Prototype Using Low-Cost, Compact Single Photon Avalanche Detectors for Tissue Optics Applications. Sensors 2018, 18, 3680. https://doi.org/10.3390/s18113680
Alayed M, Palubiak DP, Deen MJ. Characterization of a Time-Resolved Diffuse Optical Spectroscopy Prototype Using Low-Cost, Compact Single Photon Avalanche Detectors for Tissue Optics Applications. Sensors. 2018; 18(11):3680. https://doi.org/10.3390/s18113680
Chicago/Turabian StyleAlayed, Mrwan, Darek P. Palubiak, and M. Jamal Deen. 2018. "Characterization of a Time-Resolved Diffuse Optical Spectroscopy Prototype Using Low-Cost, Compact Single Photon Avalanche Detectors for Tissue Optics Applications" Sensors 18, no. 11: 3680. https://doi.org/10.3390/s18113680






