A Portable, Wireless Photoplethysomography Sensor for Assessing Health of Arteriovenous Fistula Using Class-Weighted Support Vector Machine
Abstract
:1. Introduction
2. Theories and Principles
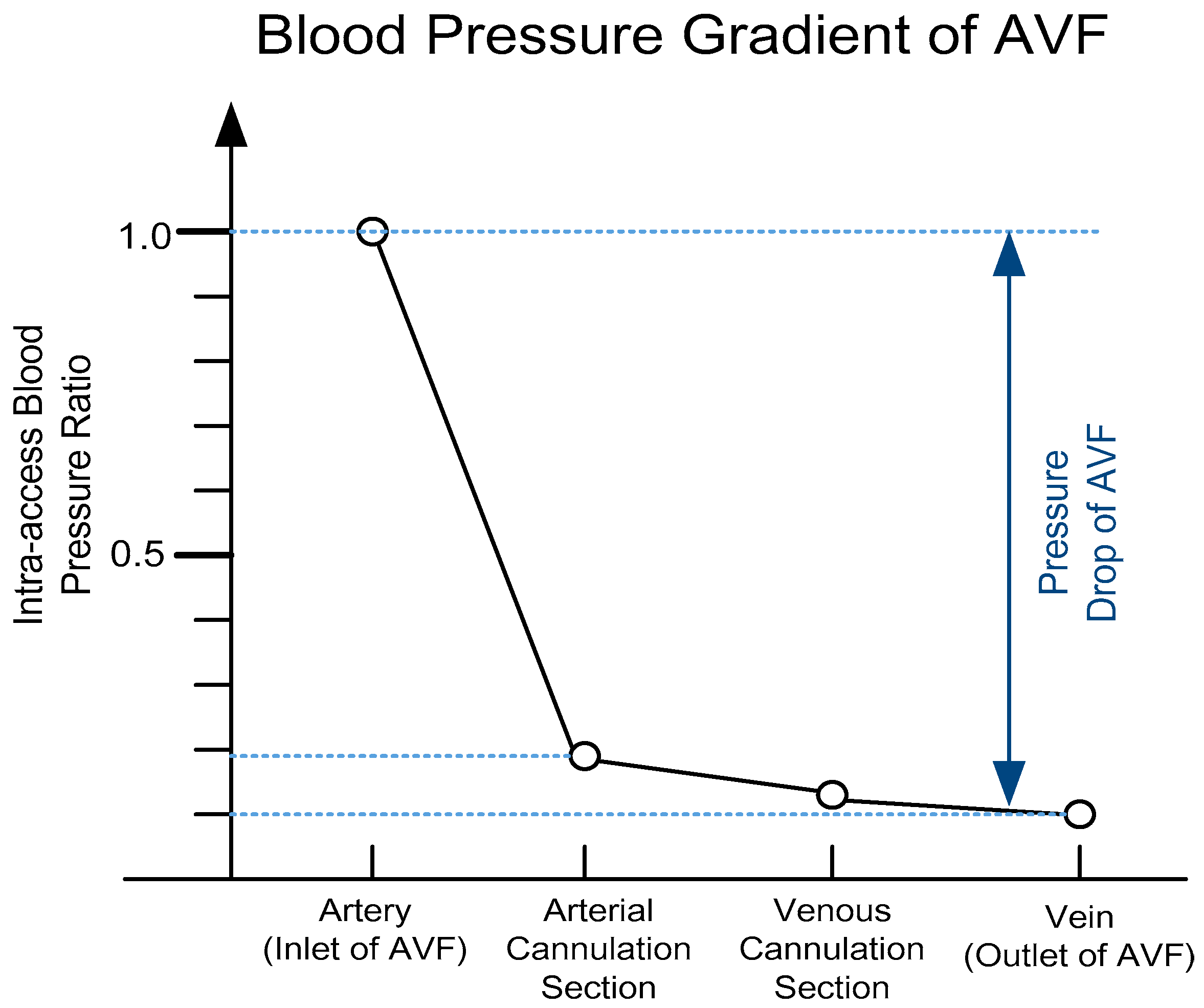
2.1. Dimensionless Analysis of Hemodynamic Model
2.2. Optical Theory of PPG Sensors
3. System Designs
3.1. Hardware Circuitries Design
3.2. Software Algorithm Design
3.2.1. Digital Signal Processing
3.2.2. Class-Weighted Support Vector Machine
- Step (1) All subjects are randomly grouped into k non-overlapping subsets.
- Step (2) One subset is tested with other k-1 subsets as the training set.
- Step (3) Step (2) repeats k times with different subsets as testing.
- Step (4) The average accuracy and error can be then calculated.
4. Clinical Validation
4.1. Experimental Setup
4.2. Experimental Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Ethical Statements
References
- National Kidney Foundation. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for 2006 Updates: Hemodialysis Adequacy, Peritoneal Dialysis Adequacy and Vascular Access. Am. J. Kidney Dis. 2006, 48, S1–S322. [Google Scholar]
- Yeih, D.F.; Wang, Y.S.; Huang, Y.C.; Chen, M.F.; Lu, S.S. Physiology-based diagnosis algorithm for arteriovenous fistula stenosis detection. In Proceedings of the 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 4619–4622. [Google Scholar] [CrossRef]
- Wang, H.Y.; Wu, C.H.; Chen, C.Y.; Lin, B.S. Novel Noninvasive Approach for Detecting Arteriovenous Fistula Stenosis. IEEE Trans. Biomed. Eng. 2014, 61, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Durand, L.G.; Pibarot, P. Digital signal processing of the phonocardiogram: Review of the most recent advances. Crit. Rev. Biomed. Eng. 1995, 23, 163–219. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.-C.; Stephanus, A. Levenberg-Marquardt Neural Network Algorithm for Degree of Arteriovenous Fistula Stenosis Classification Using a Dual Optical Photoplethysmography Sensor. Sensors 2018, 18, 2322. [Google Scholar] [CrossRef] [PubMed]
- Chiang, P.Y.; Chao, P.C.P.; Tarng, D.C.; Yang, C.Y. A Novel Wireless Photoplethysmography Blood-Flow Volume Sensor for Assessing Arteriovenous Fistula of Hemodialysis Patients. IEEE Trans. Ind. Electron. 2017, 64, 9626–9635. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, Z. Photoplethysmography-Based Heart Rate Monitoring Using Asymmetric Least Squares Spectrum Subtraction and Bayesian Decision Theory. IEEE Sens. J. 2015, 15, 7161–7168. [Google Scholar] [CrossRef]
- Temko, A. Accurate Heart Rate Monitoring During Physical Exercises Using PPG. IEEE Trans. Biomed. Eng. 2017, 64, 2016–2024. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.S.; Hyder, R.; Hafiz, M.S.B.; Haque, M.A. Real-Time Robust Heart Rate Estimation from Wrist-Type PPG Signals Using Multiple Reference Adaptive Noise Cancellation. IEEE J. Biomed. Health Inf. 2018, 22, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Alghoul, K.; Alharthi, S.; Osman, H.A.; Saddik, A.E. Heart Rate Variability Extraction from Videos Signals: ICA vs. EVM Comparison. IEEE Access 2017, 5, 4711–4719. [Google Scholar] [CrossRef]
- Morelli, D.; Bartoloni, L.; Colombo, M.; Plans, D.; Clifton, D.A. Profiling the propagation of error from PPG to HRV features in a wearable physiological-monitoring device. Healthc. Technol. Lett. 2018, 5, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Alqaraawi, A.; Alwosheel, A.; Alasaad, A. Heart rate variability estimation in photoplethysmography signals using Bayesian learning approach. Healthc. Technol. Lett. 2016, 3, 136–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.C.; Hung, P.H.; Hong, C.H.; Wang, H.M. A New Image Blood Pressure Sensor Based on PPG, RRT, BPTT, and Harmonic Balancing. IEEE Sens. J. 2014, 14, 3685–3692. [Google Scholar] [CrossRef]
- Wang, G.; Atef, M.; Lian, Y. Towards a Continuous Non-Invasive Cuffless Blood Pressure Monitoring System Using PPG: Systems and Circuits Review. IEEE Circuits Syst. Mag. 2018, 18, 6–26. [Google Scholar] [CrossRef]
- He, X.; Goubran, R.A.; Liu, X.P. Secondary Peak Detection of PPG Signal for Continuous Cuffless Arterial Blood Pressure Measurement. IEEE Trans. Instrum. Meas. 2014, 63, 1431–1439. [Google Scholar] [CrossRef]
- Budidha, K.; Rybynok, V.; Kyriacou, P.A. Design and Development of a Modular, Multichannel Photoplethysmography System. IEEE Trans. Instrum. Meas. 2018, 67, 1954–1965. [Google Scholar] [CrossRef] [Green Version]
- Yousefi, R.; Nourani, M. Separating Arterial and Venous-Related Components of Photoplethysmographic Signals for Accurate Extraction of Oxygen Saturation and Respiratory Rate. IEEE J. Biomed. Health Inform. 2015, 19, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Liu, C.; Tsow, F.; Yang, Y.; Du, Z.; Iriya, R.; Yu, H.; Tao, N. Noncontact Monitoring of Blood Oxygen Saturation Using Camera and Dual-Wavelength Imaging System. IEEE Trans. Biomed. Eng. 2016, 63, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Ghamari, M.; Soltanpur, C.; Cabrera, S.; Romero, R.; Martinek, R.; Nazeran, H. Design and prototyping of a wristband-type wireless photoplethysmographic device for heart rate variability signal analysis. In Proceedings of the 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 17–20 August 2016; pp. 4967–4970. [Google Scholar] [CrossRef]
- He, Z.; Chen, X.; Fang, Z.; Sheng, T.; Xia, S. Fusion estimation of respiration rate from ECG and PPG signal based on Android platform and wearable watch. In Proceedings of the 2nd IET International Conference on Biomedical Image and Signal Processing, Wuhan, China, 13–14 May 2017; pp. 1–6. [Google Scholar] [CrossRef]
- Lee, K.; Song, K.; Roh, T.; Yoo, H.J. A fabric wrist patch sensor for continuous and comprehensive monitoring of the cardiovascular system. In Proceedings of the 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 17–20 August 2016; pp. 6070–6073. [Google Scholar] [CrossRef]
- Wang, L.; Lo, B.P.; Yang, G. Multichannel Reflective PPG Earpiece Sensor with Passive Motion Cancellation. IEEE Trans. Biomed. Circuits Syst. 2007, 1, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.; Lee, C.C.; Chan, W.M.; Choy, S.O.; Kwok, P. Development of a wearable system integrated with novel biomedical sensors for ubiquitous healthcare. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 5802–5805. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Ko, H. Low-Power Photoplethysmogram Acquisition Integrated Circuit with Robust Light Interference Compensation. Sensors 2016, 16, 46. [Google Scholar] [CrossRef] [PubMed]
- Chiang, P.-Y.; Chao, P.C.-P.; Yang, C.-Y.; Tarng, D.-C. Theoretical Developments and Clinical Experiments of Measuring Blood Flow Volume (BFV) at Arteriovenous Fistula (AVF) Using a Photoplethysmography (PPG) Sensor. Microsyst. Technol. 2018, 1–17. [Google Scholar] [CrossRef]
- Zhang, Z.; Pi, Z.; Liu, B. TROIKA: A General Framework for Heart Rate Monitoring Using Wrist-Type Photoplethysmographic Signals During Intensive Physical Exercise. IEEE Trans. Biomed. Eng. 2015, 62, 522–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dao, D.; Salehizadeh, S.M.A.; Noh, Y.; Chong, J.W.; Cho, C.H.; McManus, D.; Darling, C.E.; Mendelson, Y.; Chon, K.H. A Robust Motion Artifact Detection Algorithm for Accurate Detection of Heart Rates from Photoplethysmographic Signals Using Time–Frequency Spectral Features. IEEE J. Biomed. Health Inform. 2017, 21, 1242–1253. [Google Scholar] [CrossRef] [PubMed]
- Wijshoff, R.W.C.G.R.; Mischi, M.; Aarts, R.M. Reduction of Periodic Motion Artifacts in Photoplethysmography. IEEE Trans. Biomed. Eng. 2017, 64, 196–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.; Cheng, Y.; Zhu, J.; Xue, D.; Abt, G.; Ye, H.; Peng, Y. A Novel Adaptive Spectrum Noise Cancellation Approach for Enhancing Heartbeat Rate Monitoring in a Wearable Device. IEEE Access 2018, 6, 8364–8375. [Google Scholar] [CrossRef]
- Yigit, B.; Pekkan, K. Non-dimensional physics of pulsatile cardiovascular networks and energy efficiency. J. R. Soc. Interface 2016, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sainas, G.; Milia, R.; Palazzolo, G.; Ibba, G.; Marongiu, E.; Roberto, S.; Pinna, V.; Ghiani, G.; Tocco, F.; Crisafulli, A. Mean Blood Pressure Assessment during Post-Exercise: Result from Two Different Methods of Calculation. J. Sports Sci. Med. 2016, 15, 424–433. [Google Scholar] [PubMed]
- Hu, S.; Shi, P.; Azorin, V.P.; Echiadis, A.; Zheng, J.; Zhu, Y.; Cheang, P.Y.S. Non-contact Reflection Photoplethysmography Towards Effective Human Physiological Monitoring. J. Med. Biol. Eng. 2010, 30, 161–168. [Google Scholar]
- Venema, B.; Gehring, H.; Michelsen, I.; Blanik, N.; Blazek, V.; Leonhardt, S. Robustness, Specificity, and Reliability of an In-Ear Pulse Oximetric Sensor in Surgical Patients. IEEE J. Biomed. Health Inform. 2014, 18, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Shin, H.S.; Park, J.; Lee, M. The Optimal Attachment Position for a Fingertip Photoplethysmographic Sensor With Low DC. IEEE Sens. J. 2012, 12, 1253–1254. [Google Scholar] [CrossRef]
- Nitzan, M.; Romem, A.; Koppel, R. Pulse oximetry: Fundamentals and technology update. Med. Devices 2014, 7, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.-Y.; Park, J.; Inoue, A. Soft Computing in Machine Learning; Springer: Zürich, Switzerland, 2014. [Google Scholar] [CrossRef]
- Osuna, E.E.; Freund, R.; Girosi, F. Support Vector Machines: Training and Applications; Technical Report; Massachusetts Institute of Technology: Cambridge, MA, USA, 1997; Volume 1602, pp. 1–42. [Google Scholar]
- Akbani, R.; Kwek, S.; Japkowicz, N. Applying support vector machines to imbalanced datasets. In Proceedings of the 15th European Conference on Machine Learning, Pisa, Italy, 20–24 September 2004; Springer: Berlin/Heidelberg, Germany, 2004; Volume 3201, pp. 39–50. [Google Scholar] [CrossRef]
- Wang, J.T.L.; Zaki, M.J.; Toivonen, H.; Shasha, D. Data Mining in Bioinformatics; Springer: London, UK, 2005. [Google Scholar]
- Gonzalez, N.F.F.; Stilianova, S.M.; Renteria, G.L.; Belanche, M.L.A.; Flores, R.B.L.; Ibarra, E.J.E. Glucose Oxidase Biosensor Modeling and Predictors Optimization by Machine Learning Methods. Sensors 2016, 16, 1483. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Yan, Y.; Wei, W.; Wang, Z.J. Classification of EEG Signals Using a Multiple Kernel Learning Support Vector Machine. Sensors 2014, 14, 12784–12802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.H.; Verma, N. A Low-Power Processor with Configurable Embedded Machine-Learning Accelerators for High-Order and Adaptive Analysis of Medical-Sensor Signals. IEEE J. Solid-State Circuits 2013, 48, 1625–1637. [Google Scholar] [CrossRef]
- Nalbantov, G.; Bioch, J.C.; Groenen, P.J.F. Data and Information Analysis to Knowledge Engineering. Studies in Classification, Data Analysis, and Knowledge Organization; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar] [CrossRef]
- Han, H.; Jiang, X. Overcome Support Vector Machine Diagnosis Overfitting. Cancer Inform. 2014, 13, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.X.; Lin, C.H.; Du, Y.C.; Chen, P.J.; Shih, C.-C.; Chen, T. Estimation of arteriovenous fistula stenosis by FPGA based Doppler flow imaging system. In Proceedings of the 2015 IEEE International Ultrasonics Symposium (IUS), Taipei, Taiwan, 21–24 October 2015; pp. 1–4. [Google Scholar] [CrossRef]











| Symbol | Measurement | Definition |
|---|---|---|
| PI | The Proposed PPG Sensor | Perfusion index defined in Equation (12) |
| SpO2 | Oximeter | Blood oxygen saturation levels |
| SBP | Electronic Sphygmomanometer | Systolic blood pressure |
| DBP | Electronic Sphygmomanometer | Diastolic blood pressure |
| fHR | The Proposed PPG Sensor | Heart rate |
| RBF Kernel | Linear Kernel | 2-nd Order Polynomial | 3-rd Order Polynomial | 4-th Order Polynomial | |
|---|---|---|---|---|---|
| Kernel Scale (σ) | 6.1585 | - | - | - | - |
| Misclassification Cost of Positive Class (C+) | 161.8024 | 1.7159 | 0.4887 | 0.1053 | 0.0603 |
| Misclassification Cost of Negative Class (C−) | 420.6862 | 4.4615 | 1.2706 | 0.2738 | 0.1567 |
| Accuracy | 89.11% | 68.32% | 75.25% | 83.17% | 87.13% |
| Sensitivity | 90.41% | 68.49% | 75.34% | 86.30% | 90.41% |
| Type II Error | 9.59% | 31.51% | 24.66% | 13.70% | 9.59% |
| RBF Kernel | Linear Kernel | 2-nd Order Polynomial | 3-rd Order Polynomial | 4-th Order Polynomial | ||
|---|---|---|---|---|---|---|
| Patients #1 | Ground Truth | P | P | P | P | P |
| First Measurement | P | P | P | P | P | |
| Second Measurement | P | P | P | P | P | |
| Third Measurement | P | P | P | P | P | |
| Accuracy | 100% | 100% | 100% | 100% | 100% | |
| Patients #2 | Ground Truth | N | N | N | N | N |
| First Measurement | N | N | N | N | N | |
| Second Measurement | N | N | N | N | N | |
| Third Measurement | N | N | N | N | N | |
| Accuracy | 100% | 100% | 100% | 100% | 100% | |
| Patients #3 | Ground Truth | P | P | P | P | P |
| First Measurement | P | N | P | P | P | |
| Second Measurement | P | N | P | P | P | |
| Third Measurement | P | N | P | P | P | |
| Accuracy | 100% | 0% | 100% | 100% | 100% | |
| Patients #4 | Ground Truth | N | N | N | N | N |
| First Measurement | N | P | P | P | N | |
| Second Measurement | N | P | N | N | N | |
| Third Measurement | N | P | N | N | N | |
| Accuracy | 100% | 0% | 66.67% | 66.67% | 100% | |
| Patients #5 | Ground Truth | P | P | P | P | P |
| First Measurement | P | P | P | P | P | |
| Second Measurement | P | P | P | P | P | |
| Third Measurement | P | P | P | P | P | |
| Accuracy | 100% | 100% | 100% | 100% | 100% |
| Wu J. X. et al. (2015) [45] | Yeih D. F. et al. (2014) [2] | Wang H. Y. et al. (2014) [3] | Du Y.-C. et al. (2018) [5] | Chiang P. Y. et al. (2017) [6] | This Work | |
|---|---|---|---|---|---|---|
| Sensor | Ultrasound | Stethoscope Auscultation | Stethoscope Auscultation | Bilateral PPG | Single PPG | Single PPG |
| Principle | Doppler | Acoustic | Acoustic | Optical | Optical | Optical |
| Communication | Wired | Wireless | Wireless | Wired | Wireless | Wireless |
| Assessing Algorithm | Color Relation Analysis | SVM | Neural Network | Neural Network | Neural Network | SVM |
| Size | Large | - | 9 cm × 4 cm × 2 cm | Large | 9 cm × 8 cm × 4 cm | 9 cm × 3.5 cm × 1.5 cm |
| Number of Subjects | 50 | 22 | 479 | 11 | 40 | 101 |
| Accuracy | 83% | 84.3% | 87.8% | 94.82% | R2 = 0.7176 | 89.11% |
| Type II Error | - | 16.7% | 10.75% | - | > 50% | 9.59% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chao, P.C.-P.; Chiang, P.-Y.; Kao, Y.-H.; Tu, T.-Y.; Yang, C.-Y.; Tarng, D.-C.; Wey, C.-L. A Portable, Wireless Photoplethysomography Sensor for Assessing Health of Arteriovenous Fistula Using Class-Weighted Support Vector Machine. Sensors 2018, 18, 3854. https://doi.org/10.3390/s18113854
Chao PC-P, Chiang P-Y, Kao Y-H, Tu T-Y, Yang C-Y, Tarng D-C, Wey C-L. A Portable, Wireless Photoplethysomography Sensor for Assessing Health of Arteriovenous Fistula Using Class-Weighted Support Vector Machine. Sensors. 2018; 18(11):3854. https://doi.org/10.3390/s18113854
Chicago/Turabian StyleChao, Paul C.-P., Pei-Yu Chiang, Yung-Hua Kao, Tse-Yi Tu, Chih-Yu Yang, Der-Cherng Tarng, and Chin-Long Wey. 2018. "A Portable, Wireless Photoplethysomography Sensor for Assessing Health of Arteriovenous Fistula Using Class-Weighted Support Vector Machine" Sensors 18, no. 11: 3854. https://doi.org/10.3390/s18113854
APA StyleChao, P. C.-P., Chiang, P.-Y., Kao, Y.-H., Tu, T.-Y., Yang, C.-Y., Tarng, D.-C., & Wey, C.-L. (2018). A Portable, Wireless Photoplethysomography Sensor for Assessing Health of Arteriovenous Fistula Using Class-Weighted Support Vector Machine. Sensors, 18(11), 3854. https://doi.org/10.3390/s18113854





