Towards an Architecture to Guarantee Both Data Privacy and Utility in the First Phases of Digital Clinical Trials
Abstract
:1. Introduction
2. Use Cases
2.1. Characterization of the Users
- R1
- The quality of data provided by participants is guaranteed (only consider data collected from approved devices).
- R2C
- Candidates are clustered on the bases of the results of a well-defined clustering algorithm automatically executed over a provided data set of historic values.
- R3
- The candidate must provide proof that the historic data set is real and collected over the stated period of time.
- R4
- Data are collected by certified and trusted devices.
- R5
- Fake data cannot be introduced into the private space.
- R6C
- Candidates’ privacy is preserved during the clustering phase. Namely, none of the users’ data are disclosed to the institute. The only information received back from the institute are the ones necessary to identify the clusters without disclosing data on the single users.
- R7
- Periodic certificates are provided to prove the authenticity, integrity, and conformance of collected data (see R3).
2.2. Patient Recruitment
- R1
- Same as that in the data clustering phase.
- R2R
- The inclusion/exclusion of a candidate is based on the execution of a well-defined recruiting test automatically executed over the provided data set of historic values. As an example, the recruiting test can be the distance of a user from a given centroid identified during the data clustering phase.
- R3
- Same as that in the data clustering phase.
- R4
- Same as that in the data clustering phase.
- R5
- Same as that in the data clustering phase.
- R6R
- Candidates’ privacy is preserved during the recruiting phase. The recruiting test is privacy-preserving, namely, it does not disclose patient’s data to the institute. Data never leave the private space of the patient unless the candidate voluntarily enrolls in the trial because s/he is eligible according to the outcome of the recruiting test (see R2).
- R7
- Same as that in the data clustering phase.
3. Privacy
- The privacy of patients and the confidentiality of health care data (prevention of unauthorized disclosure of information).
- The integrity of healthcare data (prevention of unauthorized modification of information).
- The availability of health data for authorized persons (prevention of the unauthorized or unintended withholding of information or resources).
3.1. The Role of Trust
3.2. Data Protection Regulations
- Strict limits on access and disclosure must apply to all personally identifiable health data, regardless of the form in which the information is maintained.
- All personally identifiable health records must be under an individual’s control. No personal information may be disclosed without an individual’s uncoerced, informed consent.
- Health-record information systems must be required to build-in security measures to protect personal information against both unauthorized access and misuse by authorized users.
- Employers must be denied access to personally identifiable health information on their employees and prospective employees.
- Patients must be given notice of all uses of their health information.
- Individuals must have a right of access to their own medical and financial records, including rights to copy and correct any and all information contained in those records.
- Both a private right of action and a governmental enforcement mechanism must be established to prevent or remedy wrongful disclosures or other misuses of information.
- A federal oversight system must be established to ensure compliance with privacy laws and regulations.
- Explicit Consent. Clear and definite conditions for acquiring consent from data subjects (citizens) to process data.
- Data Protection Officer. A person is appointed to handle the necessary internal recordkeeping requirements.
- Sanctions. Non-compliance can result in serious penalties.
- Territorial Scope. The directive applies to all organizations processing data from data subjects (citizens) residing in the EU, not only EU-based organizations.
- Right to Access. The data subject shall have the right to obtain from the controller confirmation as to whether or not personal data concerning her/him are being processed, and, where that is the case, to access the personal data and some other information.
- Right to Rectification. Incorrect data has to be rectified.
- Right to Be Forgotten. Data subjects have the right to request data controllers to erase their data.
- Data Portability. Data subjects have the right to request their data in a portable format, which allows one to transfer its data to another data controller.
- Data Protection by Design and by Default. Develop default privacy protection mechanisms and implement monitoring processes.
- Notification Requirements. Data breaches must be reported without undue delay.
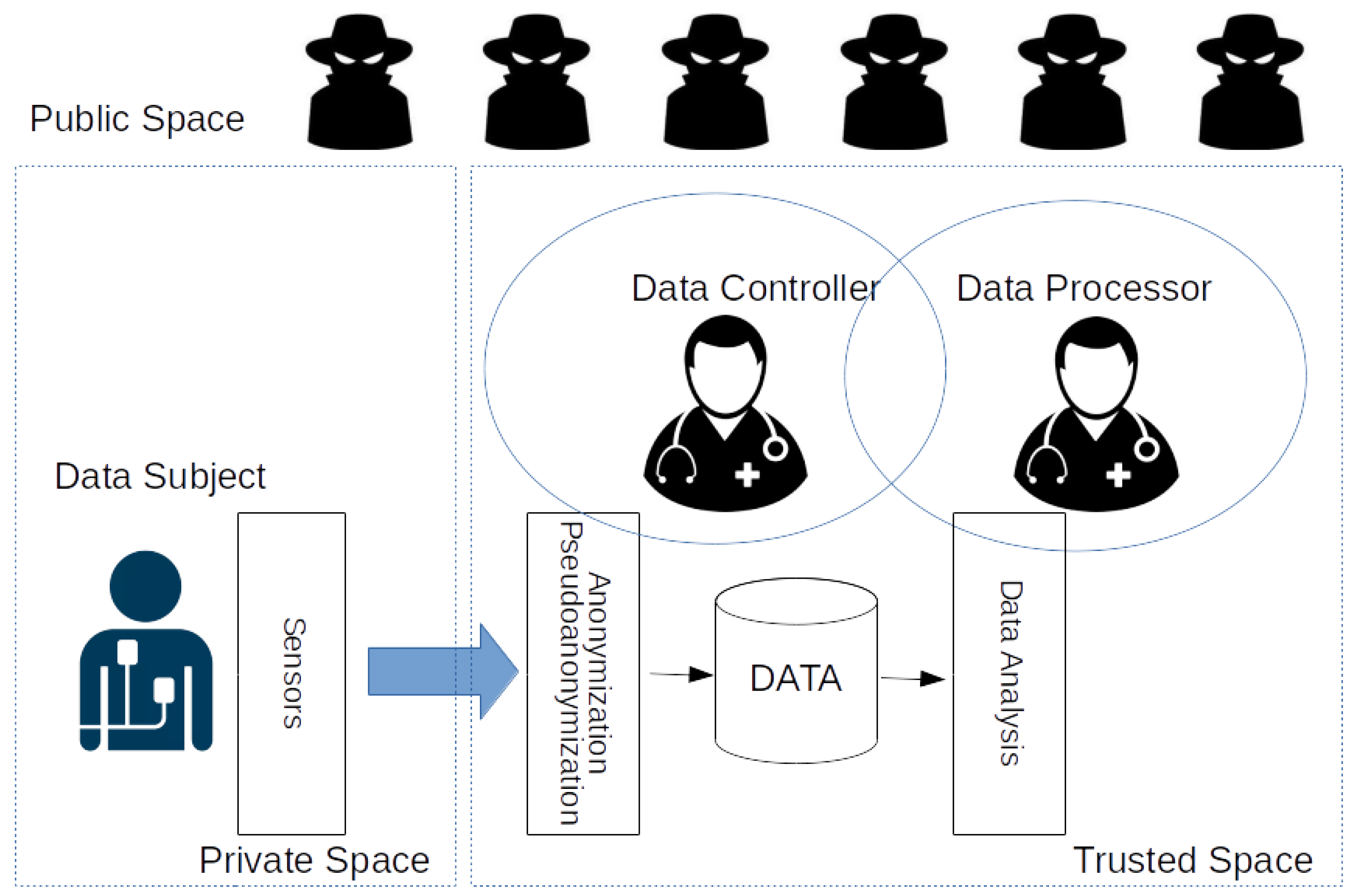
4. Data Collection in the IoHT
4.1. Accuracy
4.2. Authenticity
4.3. Confidentiality
4.4. Freshness
4.5. Availability
4.6. Integrity
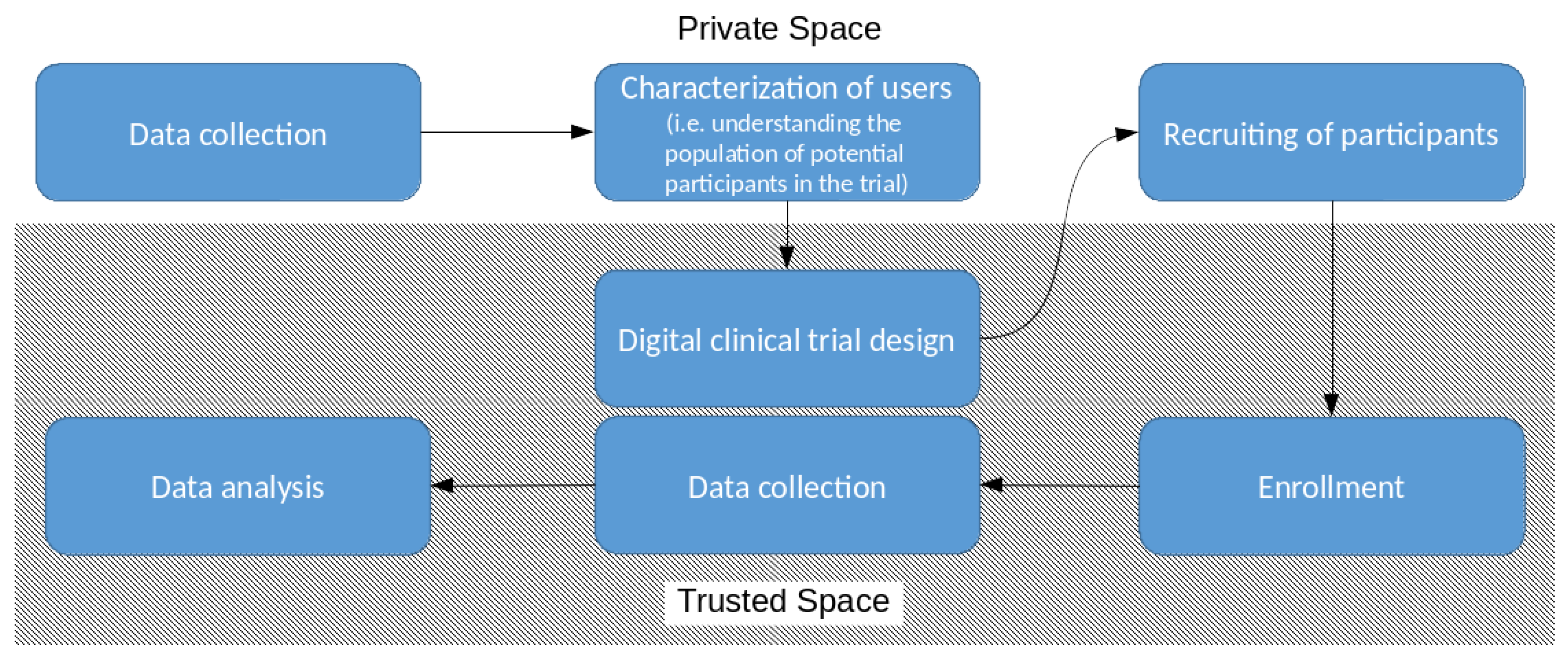
5. Characterization of Users and Recruiting of Participants in Trusted Space
5.1. k-Anonymity
5.2. l-Diversity
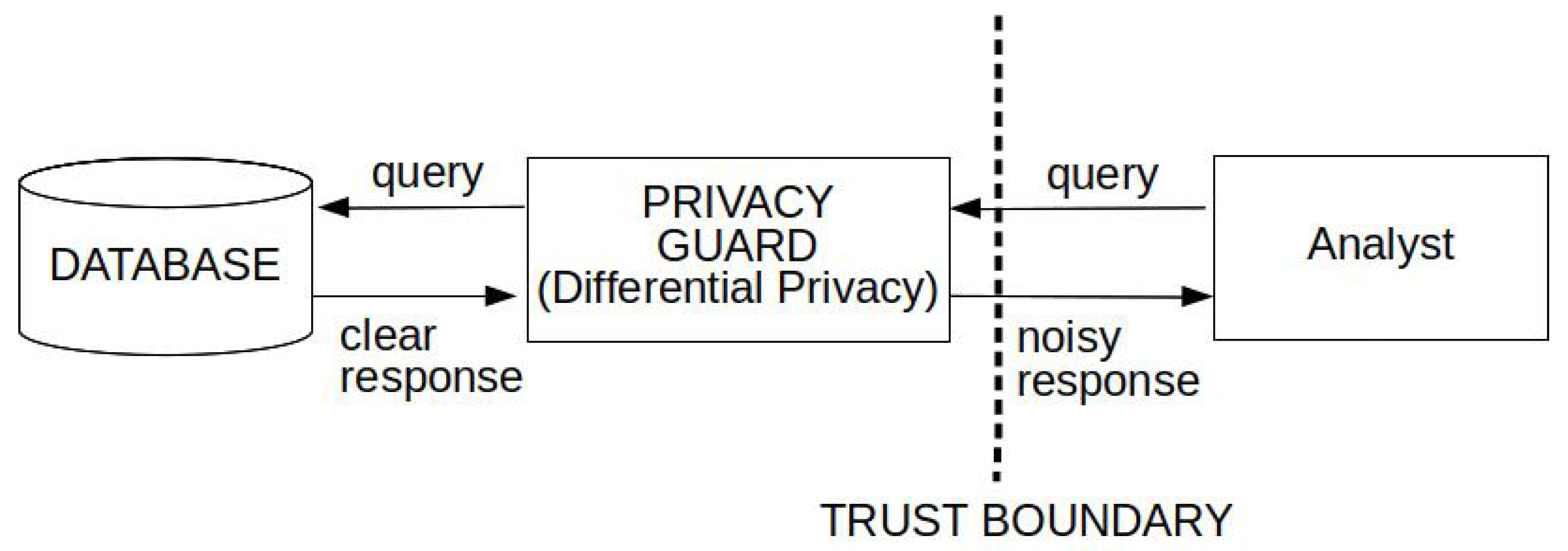
5.3. Differential Privacy
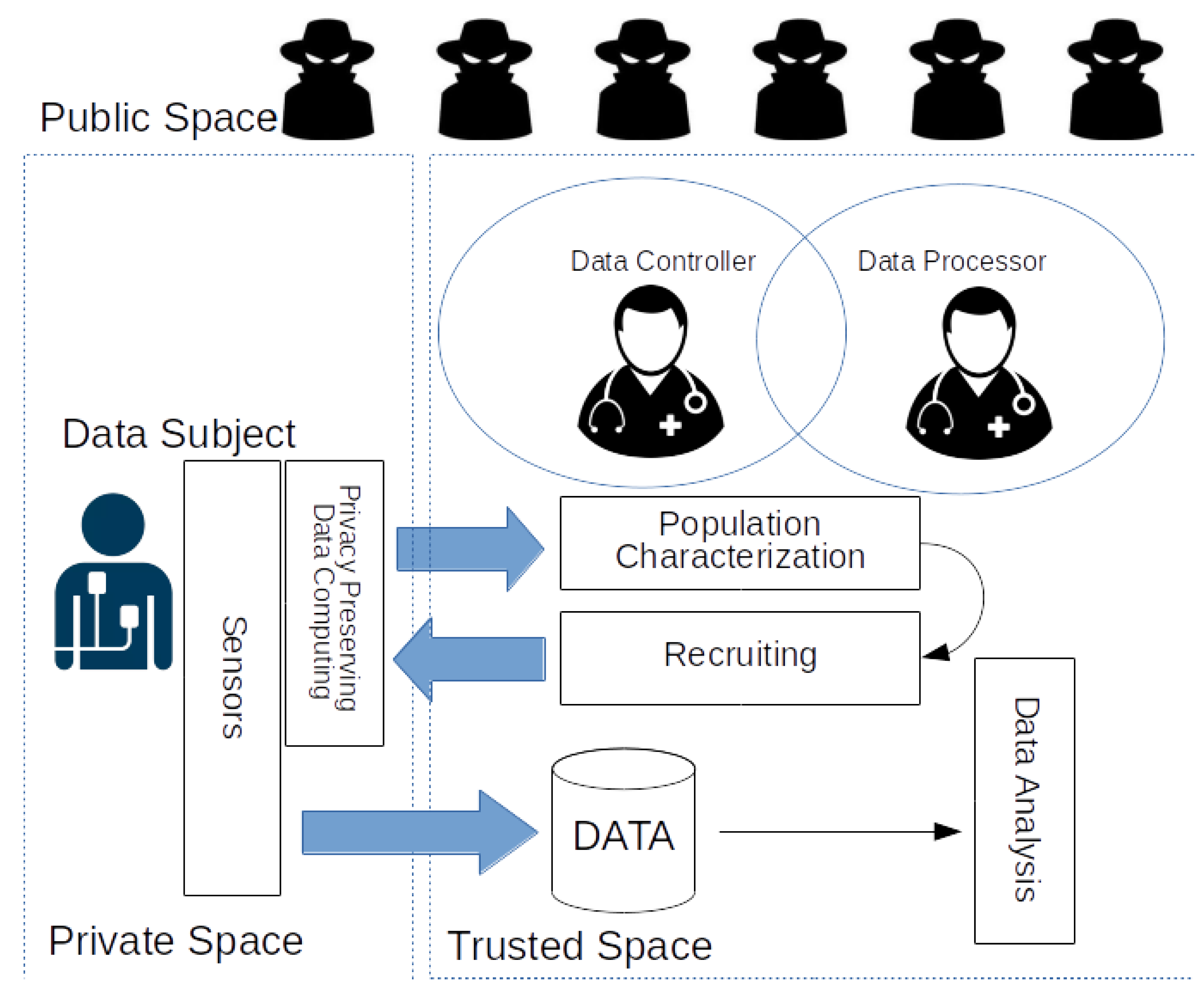
6. Characterization of Users and Recruiting of Participants in Private Space
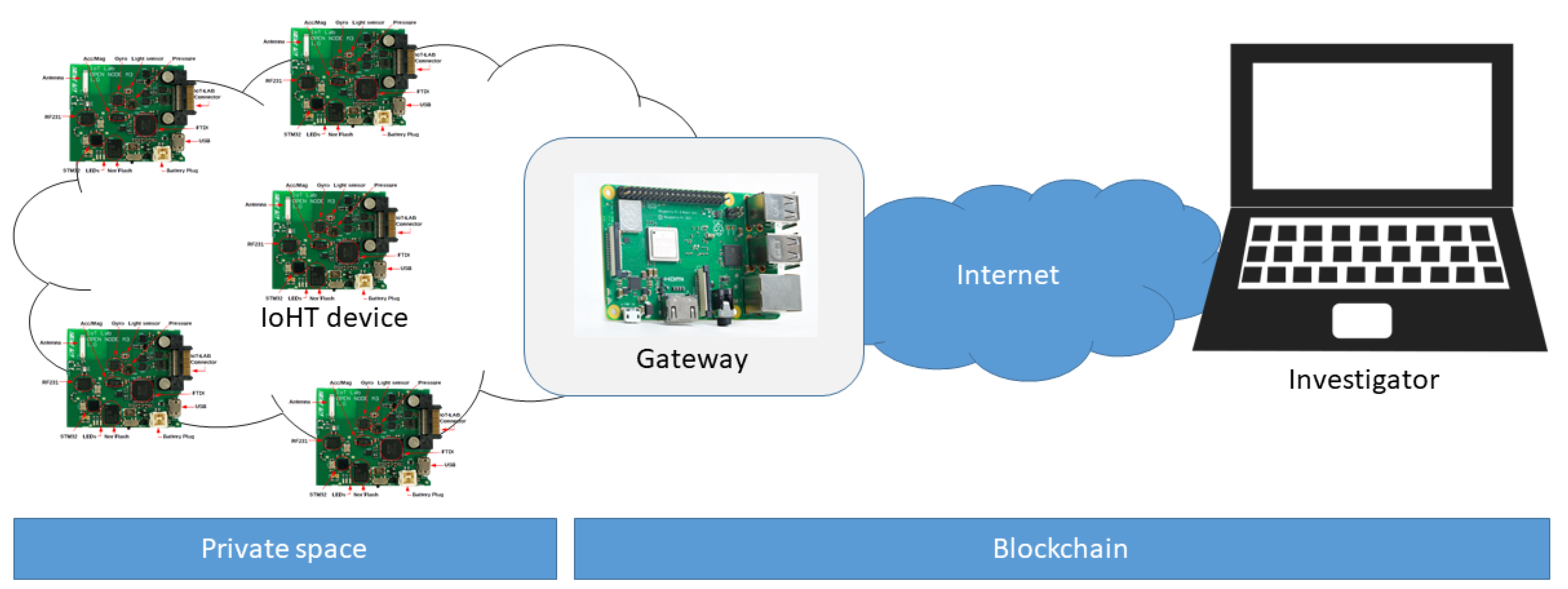
6.1. Proof of Concept
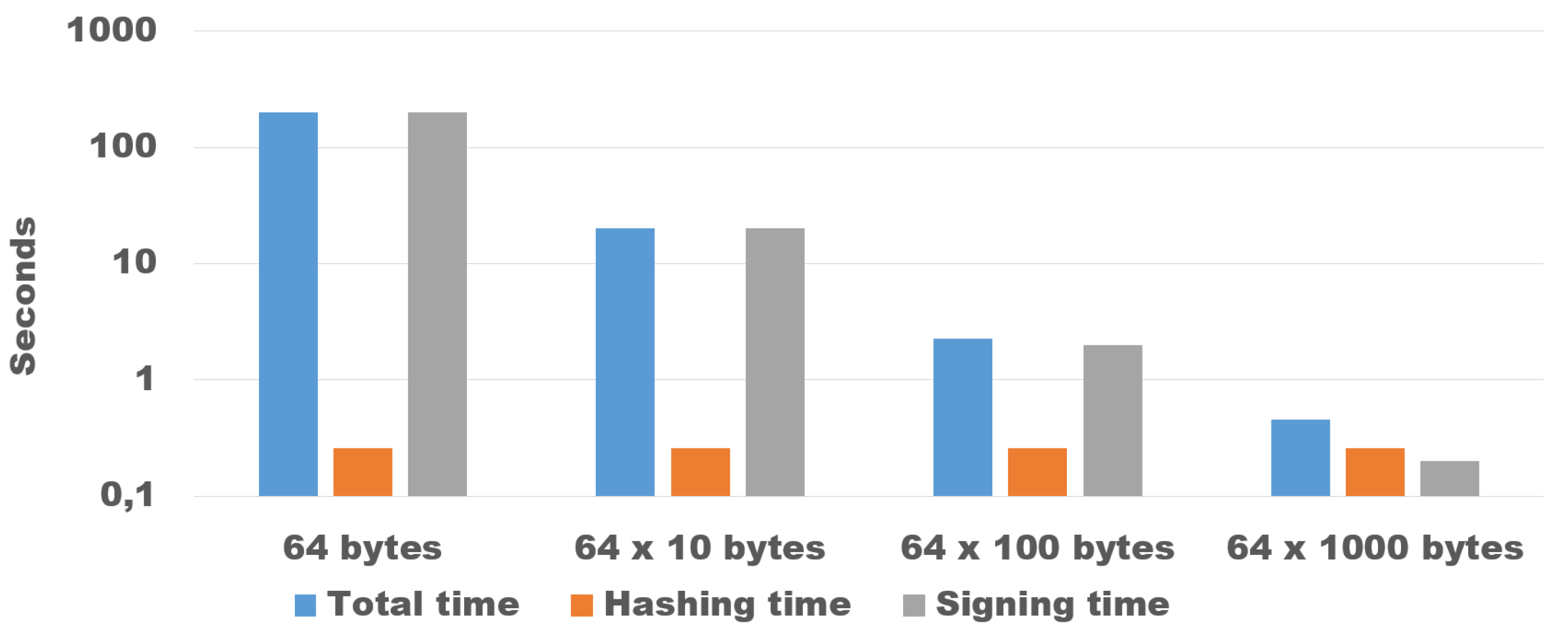
6.2. Guaranteeing Originality and Authenticity of Collected Data
- The quality of data provided by devices must be verifiable.
- Data are collected by certified and trusted devices.
- Fake data cannot be introduced into the private space.
6.3. Characterization of Potential Participants
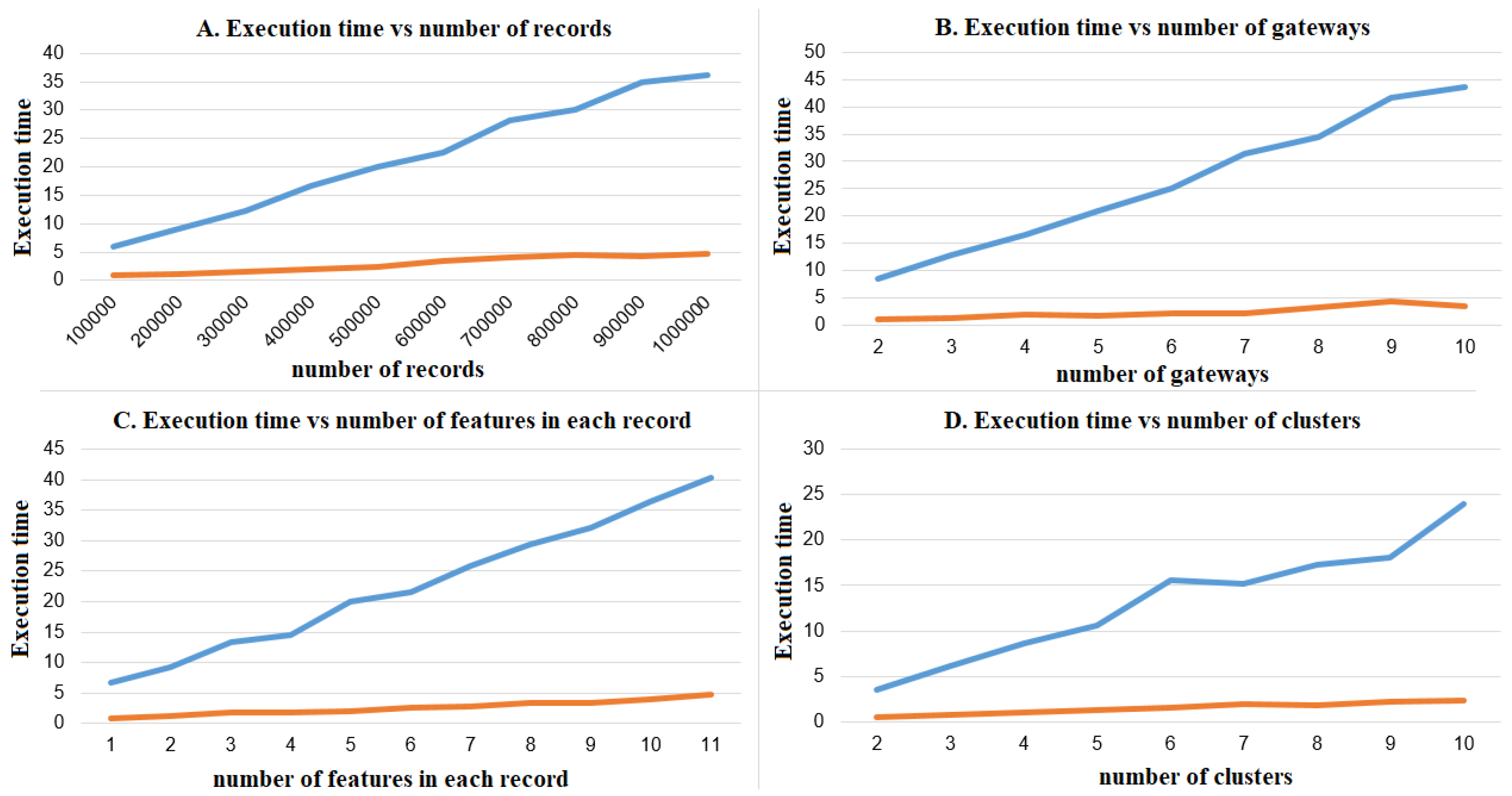
6.4. Recruiting Patients
- The inclusion/exclusion of a candidate is based on the result of a well-defined recruiting test automatically executed in the private space over a data set of genuine historic values.
- An individual that wishes to be considered for a specific clinical trial expects that the privacy of her/his personal data will be respected and the confidentiality of the private data will be guaranteed. If during the recruiting phase the individual is excluded, then the digital health system should guarantee that no personal data are retained by the investigator.
- The class of basic recruiting tests, where the recruiting test receives as input the raw data in the private space, possibly pre-processing it to extract relevant features. As an example, the recruiting test can be a threshold on the average heart-rate.
- The class of advanced recruiting tests, where a machine learning algorithm on the gateway is trained with the raw data in the private space as the training set. The recruiting test is computed over the output of the trained machine learning algorithm receiving as input a test set provided by the investigator.
7. State of the Art
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jara, A.J.; Zamora, M.A.; Skarmeta, A.F.G. An internet of things–based personal device for diabetes therapy management in ambient assisted living (AAL). Personal Ubiquit. Comput. 2011, 15, 431–440. [Google Scholar] [CrossRef]
- Hay, M.; Thomas, D.W.; Craighead, J.L.; Celia, E.; Rosenthal, J. Clinical development success rates for investigational drugs. Nat. Biotechnol. 2014, 32, 40–51. [Google Scholar] [CrossRef] [PubMed]
- DiMasi, J.A.; Grabowski, H.G.; Hansen, R.W. Innovation in the pharmaceutical industry: New estimates of R&D costs. J. Health Econ. 2016, 47, 20–33. [Google Scholar] [PubMed] [Green Version]
- Trends, Charts, and Maps at ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/resources/trends (accessed on 1 September 2018).
- The Center for Information and Study on Clinical Research Participation (CISCRP). Available online: https://www.ciscrp.org (accessed on 1 November 2018).
- Pickard, K.T.; Swan, M. Big Desire to Share Big Health Data: A Shift in Consumer Attitudes toward Personal Health Information. In Proceedings of the 2014 AAAI Spring Symposium Series, Palo Alto, CA, USA, 24–26 March 2014. [Google Scholar]
- Pavlou, P.A. State of the information privacy literature: Where are we now and where should we go? MIS Q. 2011, 35, 977–988. [Google Scholar] [CrossRef]
- Price, B.A.; Adam, K.; Nuseibeh, B. Keeping ubiquitous computing to yourself: A practical model for user control of privacy. Int. J. Hum.-Comput. Stud. 2005, 63, 228–253. [Google Scholar] [CrossRef] [Green Version]
- On Strategies for Responsible Sharing of Clinical Trial Data; Board on Health Sciences Policy; Institute of Medicine. Sharing Clinical Trial Data: Maximizing Benefits, Minimizing Risk; Guiding Principles for Sharing Clinical Trial Data; National Academies Press (US): Washington, DC, USA, 2015. Available online: https://www.ncbi.nlm.nih.gov/books/NBK285999/ (accessed on 1 November 2018).
- Terry, S.F.; Terry, P.F. Power to the People: Participant Ownership of Clinical Trial Data. Sci. Transl. Med. 2011, 3, 69cm3. [Google Scholar] [CrossRef] [PubMed]
- My Data Is Mine Declaration. Available online: http://www.mydataismine.com/manifest (accessed on 1 November 2018).
- Smith, A. U.S. Smartphone Use in 2015. PewResearchCenter. Available online: http://www.pewinternet.org/2015/04/01/us-smartphone-use-in-2015/ (accessed on 1 April 2015).
- Edney, A.; Chen, C. Big Pharma Hands Out Fitbits to Collect Better Personal Data. Bloomberg. Available online: http://www.bloomberg.com/news/articles/2015-09-14/big-pharma-hands-out-fitbits-to-collect-better-personal-data (accessed on 14 September 2015).
- Bergström, A. Online privacy concerns: A broad approach to understanding the concerns of different groups for different uses. Comput. Hum. Behav. 2015, 53, 419–426. [Google Scholar] [CrossRef]
- Preibusch, S. Guide to measuring privacy concern: Review of survey and observational instruments. Int. J. Hum.-Comput. Stud. 2013, 71, 1133–1143. [Google Scholar] [CrossRef]
- Turow, J.; Hennessy, M. Internet privacy and institutional trust: Insights from a national survey. New Media Soc. 2007, 9, 300–318. [Google Scholar] [CrossRef]
- Woo, J. The right not to be identified: Privacy and anonymity in the interactive media environment. New Media Soc. 2006, 8, 949–967. [Google Scholar] [CrossRef]
- Park, Y.J.; Campbell, S.W.; Kwak, N. Affect, cognition and reward: Predictors of privacy protection online. Comput. Hum. Behav. 2012, 28, 1019–1027. [Google Scholar] [CrossRef]
- Trepte, S.; Dienlin, T.; Reinecke, L. Privacy, Self-Disclosure, Social Support, and Social Network Site Use; University of Hohenheim: Stuttgart, Germany, 2013. [Google Scholar]
- Youn, S. Determinants of online privacy concern and its influence on privacy protection behaviors among young adolescents. J. Consum. Aff. 2009, 43, 389–418. [Google Scholar] [CrossRef]
- Westin, A. Improving Access and Protecting Privacy. Connecting Americans to Their Health Care. 2006. Available online: http://www. phrconference.org/conf_resources/presentations/dec7/improving_access.pdf (accessed on 2 March 2007).
- Bansal, G.; Zahedi, F.M.; Gefen, D. The Impact of Personal Dispositions on Information Sensitivity, Privacy Concern and Trust in Disclosing Health Information Online. Decis. Support Syst. 2010, 49, 138–150. [Google Scholar] [CrossRef]
- Luck, J.; Chang, C.; Brown, E.R.; Lumpkin, J. Using local health information to promote public health. Health Aff. 2006, 25, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Barrows, R.C., Jr.; Clayton, P.D. Privacy, Confidentiality, and Electronic Medical Records. J. Am. Med. Inform. Assoc. 1996, 3, 139–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lincoln, T. Privacy: A real-world problem with fuzzy boundaries. Methods Inf. Med. 1993, 32, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Westin, A.F. Privacy and Freedom; Atheneum Press: New York, NY, USA, 1967; Volume 7. [Google Scholar]
- IAPP. IAPP Information Privacy Certification, Glossary of Common Privacy Terminology; IAPP: Portsmouth, NH, USA, 2011. [Google Scholar]
- Steinfeld, L.; Archuleta, K.S. Privacy Protection and Compliance in Higher Education: The Role of the CPO. Educ. Rev. 2006, 41, 62. [Google Scholar]
- Milberg, S.J.; Smith, H.J.; Burke, S.J. Information privacy: Corporate management and national regulation. Organ. Sci. 2000, 11, 35–57. [Google Scholar] [CrossRef]
- Okazaki, S.; Li, H.; Hirose, M. Consumer privacy concerns and preference for degree of regulatory control. J. Advert. 2009, 38, 63–77. [Google Scholar] [CrossRef]
- Smith, H.J.; Milberg, S.J.; Burke, S.J. Information privacy: Measuring individuals’ concerns about organizational practices. MIS Q. 1996, 20, 167–196. [Google Scholar] [CrossRef]
- Wirtz, J.; Lwin, M.O.; Williams, J.D. Causes and consequences of consumer online privacy concern. Int. J. Serv. Ind. Manag. 2007, 18, 326–348. [Google Scholar] [CrossRef]
- Lavagnino, M.B. Information Privacy Revealed. Educ. Rev. 2013, 48, 10. [Google Scholar]
- Bakker, A. Security in medical information systems. Yearb. Med. Inform. 1993, 2, 52–60. [Google Scholar] [CrossRef]
- Bengtsson, S. Clinical requirements for the security of the electronic patient record. Int. J. Bio-Med. Comput. 1994, 35, 29–31. [Google Scholar]
- Bodenheimer, T.; Grumbach, K. Electronic technology: A spark to revitalize primary care? JAMA 2003, 290, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Cantor, J.D. Privacy protections for cybercharts: An update on the law. JAMA 2001, 285, 1767. [Google Scholar] [CrossRef] [PubMed]
- Masys, D.; Baker, D.; Butros, A.; Cowles, K.E. Giving patients access to their medical records via the internet: The PCASSO experience. J. Am. Med. Inform. Assoc. 2002, 9, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Shortliffe, E.H. Strategic action in health information technology: Why the obvious has taken so long. Health Aff. 2005, 24, 1222–1233. [Google Scholar] [CrossRef] [PubMed]
- Stewart, K.A.; Segars, A.H. An empirical examination of the concern for information privacy instrument. Inf. Syst. Res. 2002, 13, 36–49. [Google Scholar] [CrossRef]
- Lazarou, J.; Pomeranz, B.H.; Corey, P.N. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA 1998, 279, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S. Determinants of long-term orientation in buyer-seller relationships. J. Mark. 1994, 58, 1–19. [Google Scholar] [CrossRef]
- Mayer, R.C.; Davis, J.H.; Schoorman, F.D. An integrative model of organizational trust. Acad. Manag. Rev. 1995, 20, 709–734. [Google Scholar] [CrossRef]
- McKnight, D.H.; Choudhury, V.; Kacmar, C. Developing and validating trust measures for e-commerce: An integrative typology. Inf. Syst. Res. 2002, 13, 334–359. [Google Scholar] [CrossRef]
- Fukuyama, F. Trust: The Social Virtues and the Creation of Prosperity; D10 301 c.1/c.2; Free Press Paperbacks: New York, NY, USA, 1995. [Google Scholar]
- Schlichter, B.R.; Rose, J. Trust dynamics in a large system implementation: Six theoretical propositions. Eur. J. Inf. Syst. 2013, 22, 455–474. [Google Scholar] [CrossRef]
- Dinev, T.; Bellotto, M.; Hart, P.; Russo, V.; Serra, I.; Colautti, C. Privacy calculus model in e-commerce—A study of Italy and the United States. Eur. J. Inf. Syst. 2006, 15, 389–402. [Google Scholar] [CrossRef]
- Jøsang, A. The right type of trust for distributed systems. In Proceedings of the 1996 Workshop on New Security Paradigms, Lake Arrowhead, CA, USA, 17–20 September 1996; pp. 119–131. [Google Scholar]
- Union, A.C.L. Toward a New Health Care System: The Civil Liberties Issues; Technical Report, An ACLU Public Policy Report (ISBN O-914031-24-4); ACLU: New York, NY, USA, 1994. [Google Scholar]
- Ni, L.M.; Zhang, Q.; Tan, H.; Luo, W.; Tang, X. Smart healthcare: From IoT to cloud computing. Sci. Sin. Inf. 2013, 43, 515–528. [Google Scholar]
- Lima, L.; Novais, P.; Costa, R.; Cruz, J.B.; Neves, J. Group decision making and Quality-of-Information in e-Health systems. Logic J. IGPL 2011, 19, 315–332. [Google Scholar] [CrossRef] [Green Version]
- El-Amrawy, F.; Nounou, M.I. Are currently available wearable devices for activity tracking and heart rate monitoring accurate, precise, and medically beneficial? Healthc. Inf. Res. 2015, 21, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Adam Noah, J.; Spierer, D.K.; Gu, J.; Bronner, S. Comparison of steps and energy expenditure assessment in adults of Fitbit Tracker and Ultra to the Actical and indirect calorimetry. J. Med. Eng. Technol. 2013, 37, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M. Validity of Consumer-Based Physical Activity Monitors and Calibration of Smartphone for Prediction of Physical Activity Energy Expenditure. Graduate Theses and Dissertations, Iowa State University, Ames, IA, USA, 2013. [Google Scholar]
- Olguın, D.O.; Pentland, A.S. Human activity recognition: Accuracy across common locations for wearable sensors. In Proceedings of the 10th IEEE International Symposium on Wearable Computers, Montreux, Switzerland, 11–14 October 2006; pp. 11–14. [Google Scholar]
- Zhou, Y.; Leri, F. Neuroscience of opiates for addiction medicine: From stress-responsive systems to behavior. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2016; Volume 223, pp. 237–251. [Google Scholar]
- Kothmayr, T.; Schmitt, C.; Hu, W.; Brünig, M.; Carle, G. DTLS based security and two-way authentication for the Internet of Things. Ad Hoc Netw. 2013, 11, 2710–2723. [Google Scholar] [CrossRef]
- Pereira, P.P.; Eliasson, J.; Delsing, J. An authentication and access control framework for CoAP-based Internet of Things. In Proceedings of the 40th IEEE Annual Conference on Industrial Electronics Society, Dallas, TX, USA, 29 October–1 November 2014; pp. 5293–5299. [Google Scholar]
- Porambage, P.; Schmitt, C.; Kumar, P.; Gurtov, A.; Ylianttila, M. Two-phase authentication protocol for wireless sensor networks in distributed IoT applications. In Proceedings of the IEEE Wireless Communications and Networking Conference (WCNC), Istanbul, Turkey, 6–9 April 2014; pp. 2728–2733. [Google Scholar]
- Mahajan, P.; Sachdeva, A. A study of encryption algorithms AES, DES and RSA for security. Glob. J. Comput. Sci. Technol. 2013, 13, 15-E. [Google Scholar]
- Prasithsangaree, P.; Krishnamurthy, P. Analysis of energy consumption of RC4 and AES algorithms in wireless LANs. In Proceedings of the Global Telecommunications Conference, Berkeley, CA, USA, 2–4 June 2003; Volume 3, pp. 1445–1449. [Google Scholar]
- Suo, H.; Wan, J.; Zou, C.; Liu, J. Security in the internet of things: A review. In Proceedings of the 2012 International Conference on Computer Science and Electronics Engineering (ICCSEE), Hangzhou, China, 23–25 March 2012; Volume 3, pp. 648–651. [Google Scholar]
- Doukas, C.; Maglogiannis, I.; Koufi, V.; Malamateniou, F.; Vassilacopoulos, G. Enabling data protection through PKI encryption in IoT m-Health devices. In Proceedings of the 12th IEEE International Conference on Bioinformatics & Bioengineering (BIBE), Larnaca, Cyprus, 11–13 November 2012; pp. 25–29. [Google Scholar]
- Yao, X.; Chen, Z.; Tian, Y. A lightweight attribute-based encryption scheme for the Internet of Things. Future Gener. Comput. Syst. 2015, 49, 104–112. [Google Scholar] [CrossRef]
- Akribopoulos, O.; Chatzigiannakis, I.; Tselios, C.; Antoniou, A. On the Deployment of Healthcare Applications over Fog Computing Infrastructure. In Proceedings of the 41st IEEE Annual Computer Software and Applications Conference (COMPSAC 2017), Turin, Italy, 4–8 July 2017; Volume 2, pp. 288–293. [Google Scholar]
- Akrivopoulos, O.; Amaxilatis, D.; Antoniou, A.; Chatzigiannakis, I. Design and Evaluation of a Person-Centric Heart Monitoring System over Fog Computing Infrastructure. In Proceedings of the First International Workshop on Human-Centered Sensing, Networking, and Systems, Delft, The Netherlands, 5 November 2017; pp. 25–30. [Google Scholar]
- Bonetto, R.; Bui, N.; Lakkundi, V.; Olivereau, A.; Serbanati, A.; Rossi, M. Secure communication for smart IoT objects: Protocol stacks, use cases and practical examples. In Proceedings of the 2012 IEEE International Symposium on World of Wireless, Mobile and Multimedia Networks (WoWMoM), San Francisco, CA, USA, 25–28 June 2012; pp. 1–7. [Google Scholar]
- Canetti, R.; Krawczyk, H. Analysis of key-exchange protocols and their use for building secure channels. In International Conference on the Theory and Applications of Cryptographic Techniques; Springer: Berlin, Germany, 2001; pp. 453–474. [Google Scholar]
- Schwabe, P.; Stoffelen, K. All the AES you need on Cortex-M3 and M4. In International Conference on Selected Areas in Cryptography; Springer: Berlin, Germany, 2016; pp. 180–194. [Google Scholar]
- Man, A.S.; Zhang, E.S.; Lau, V.K.; Tsui, C.Y.; Luong, H.C. Low power VLSI design for a RFID passive tag baseband system enhanced with an AES cryptography engine. In Proceedings of the 1st Annual RFID Eurasia, Istanbul, Turkey, 5–6 September 2007; pp. 1–6. [Google Scholar]
- Miao, F.; Cheng, Y.; He, Y.; He, Q.; Li, Y. A Wearable Context-Aware ECG Monitoring System Integrated with Built-in Kinematic Sensors of the Smartphone. Sensors 2015, 15, 11465–11484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatzigiannakis, I.; Valchinov, E.S.; Antoniou, A.; Kalogeras, A.P.; Alexakos, C.E.; Konstantinopoulos, P. Advanced observation and telemetry heart system utilizing wearable ECG device and a Cloud platform. In Proceedings of the IEEE Symposium on Computers and Communication (ISCC 2015), Larnaca, Cyprus, 6–9 July 2015; pp. 25–30. [Google Scholar]
- Phillips, R.; McGarraugh, G.; Jurik, F.A.; Underwood, R.D. Automatic Initiation of a Time Interval for Measuring Glucose Concentration in a Sample of Whole Blood. U.S. Patent 5,843,692, 1 December 1998. [Google Scholar]
- Chatzigiannakis, I.; Dimitriou, T.; Nikoletseas, S.; Spirakis, P. A Probabilistic Forwarding Protocol for Efficient Data Propagation in Sensor Networks. J. Ad Hoc Netw. 2006, 4, 621–635. [Google Scholar] [CrossRef]
- Jara, A.J.; Zamora-Izquierdo, M.A.; Skarmeta, A.F. Interconnection framework for mHealth and remote monitoring based on the internet of things. IEEE J. Sel. Areas Commun. 2013, 31, 47–65. [Google Scholar] [CrossRef]
- Lakshman, T.; Madhow, U. The performance of TCP/IP for networks with high bandwidth-delay products and random loss. IEEE/ACM Trans. Netw. (ToN) 1997, 5, 336–350. [Google Scholar] [CrossRef] [Green Version]
- Chan, M.C.; Ramjee, R. TCP/IP performance over 3G wireless links with rate and delay variation. Wirel. Netw. 2005, 11, 81–97. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, R.; Chen, Q.; Liu, Y.; Qin, W. IoT gateway: Bridgingwireless sensor networks into internet of things. In Proceedings of the IEEE/IFIP 8th International Conference on Embedded and Ubiquitous Computing (EUC), Hong Kong, China, 11–13 December 2010; pp. 347–352. [Google Scholar]
- Datta, S.K.; Bonnet, C.; Nikaein, N. An IoT gateway centric architecture to provide novel M2M services. In Proceedings of the 2014 IEEE World Forum on Internet of Things (WF-IoT), Seoul, Korea, 6–8 March 2014; pp. 514–519. [Google Scholar]
- Chen, H.; Jia, X.; Li, H. A brief introduction to IoT gateway. In Proceedings of the IET International Conference on Communication Technology and Application (ICCTA 2011), Beijing, China, 14–16 October 2011; pp. 610–613. [Google Scholar]
- Shang, G.; Chen, Y.; Zuo, C.; Zhu, Y. Design and implementation of a smart IoT gateway. In Proceedings of the IEEE International Conference on Green Computing and Communications and IEEE Internet of Things and IEEE Cyber, Physical and Social Computing, Beijing, China, 20–23 August 2013; pp. 720–723. [Google Scholar]
- Akribopoulos, O.; Chatzigiannakis, I.; Koninis, C.; Theodoridis, E. A Web Services-oriented Architecture for Integrating Small Programmable Objects in the Web of Things. In Proceedings of the 2010 Developments in E-systems Engineering, London, UK, 6–8 September 2010; pp. 70–75. [Google Scholar]
- Chen, J.; Ma, H. Efficient decentralized attribute-based access control for cloud storage with user revocation. In Proceedings of the IEEE International Conference on Communications (ICC), Sydney, Australia, 10–14 June 2014; pp. 3782–3787. [Google Scholar]
- Ruj, S.; Stojmenovic, M.; Nayak, A. Decentralized access control with anonymous authentication of data stored in clouds. IEEE Trans. Parallel Distrib. Syst. 2014, 25, 384–394. [Google Scholar] [CrossRef]
- Rahmani, A.M.; Gia, T.N.; Negash, B.; Anzanpour, A.; Azimi, I.; Jiang, M.; Liljeberg, P. Exploiting smart e-Health gateways at the edge of healthcare Internet-of-Things: A fog computing approach. Future Gener. Comput. Syst. 2018, 78, 641–658. [Google Scholar] [CrossRef]
- Chatzigiannakis, I.; Hasemann, H.; Karnstedt, M.; Kleine, O.; Kröller, A.; Leggieri, M.; Pfisterer, D.; Römer, K.; Truong, C. True self-configuration for the IoT. In Proceedings of the 3rd IEEE International Conference on the Internet of Things (IOT 2012), Wuxi, China, 24–26 October 2012; pp. 9–15. [Google Scholar]
- Chatzigiannakis, I.; Kinalis, A.; Nikoletseas, S. Power conservation schemes for energy efficient data propagation in heterogeneous wireless sensor networks. In Proceedings of the 38th Annual Simulation Symposium, San Diego, CA, USA, 4–6 April 2005; pp. 60–71. [Google Scholar]
- Chatzigiannakis, I.; Kinalis, A.; Nikoletseas, S. An Adaptive Power Conservation Scheme for Heterogeneous Wireless Sensor Networks with Node Redeployment. In Proceedings of the Seventeenth Annual ACM Symposium on Parallelism in Algorithms and Architectures, Las Vegas, NV, USA, 18–20 July 2005; ACM: New York, NY, USA, 2005; pp. 96–105. [Google Scholar]
- Adelantado, F.; Vilajosana, X.; Tuset-Peiro, P.; Martinez, B.; Melia-Segui, J.; Watteyne, T. Understanding the limits of LoRaWAN. IEEE Commun. Mag. 2017, 55, 34–40. [Google Scholar] [CrossRef]
- Luvisotto, M.; Tramarin, F.; Vangelista, L.; Vitturi, S. On the Use of LoRaWAN for Indoor Industrial IoT Applications. Wirel. Commun. Mob. Comput. 2018, 2018, 3982646. [Google Scholar] [CrossRef]
- Marais, J.M.; Malekian, R.; Abu-Mahfouz, A.M. LoRa and LoRaWAN testbeds: A review. In Proceedings of the 2017 IEEE AFRICON, Cape Town, South Africa, 18–20 September 2017; pp. 1496–1501. [Google Scholar]
- Chatzigiannakis, I.; Liagkou, V.; Spirakis, P.G. Brief Announcement: Providing End-to-End Secure Communication in Low-Power Wide Area Networks. In Proceedings of the Cyber Security Cryptography and Machine Learning, Beer Sheva, Israel, 21–22 June 2018; pp. 101–104. [Google Scholar]
- Vejlgaard, B.; Lauridsen, M.; Nguyen, H.; Kovács, I.Z.; Mogensen, P.; Sorensen, M. Coverage and capacity analysis of sigfox, lora, gprs, and nb-iot. In Proceedings of the 85th IEEE Vehicular Technology Conference (VTC Spring), Sydney, Australia, 4–7 June 2017; pp. 4–7. [Google Scholar]
- Zuniga, J.C.; Ponsard, B. Sigfox system description. In Proceedings of the LPWAN@ IETF97, Seoul, Korea, 14 November 2016. [Google Scholar]
- Lauridsen, M.; Nguyen, H.; Vejlgaard, B.; Kovács, I.Z.; Mogensen, P.; Sorensen, M. Coverage Comparison of GPRS, NB-IoT, LoRa, and SigFox in a 7800 km2 Area. In Proceedings of the 85th IEEE Vehicular Technology Conference (VTC Spring), Sydney, Australia, 4–7 June 2017; pp. 1–5. [Google Scholar]
- Lee, S.Y.; Hong, J.H.; Hsieh, C.H.; Liang, M.C.; Chien, S.Y.C.; Lin, K.H. Low-power wireless ECG acquisition and classification system for body sensor networks. IEEE J. Biomed. Health Inform. 2015, 19, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Miao, F.; Zhang, J.; Zhou, G.; Gu, L.; He, T.; Stankovic, J.A.; Son, S.; Pappas, G.J. ATPC: Adaptive transmission power control for wireless sensor networks. ACM Trans. Sens. Netw. (TOSN) 2016, 12, 6. [Google Scholar] [CrossRef]
- Angeletti, F.; Paoli, M.; Colesanti, U.M.; Vitaletti, A. Wireless sensor networks in structural health monitoring: A modular approach. In Proceedings of the 9th International Conference on Sensor Technologies and Applications (SENSORCOMM’2015), Venice, Italy, 23–28 August 2015; pp. 77–80. [Google Scholar]
- Angeletti, F.; Paoli, M.; Colesanti, U.M.; Vitaletti, A. A Modular Design for Wireless Structural Health Monitoring Applications. Sens. Transducers 2015, 194, 134. [Google Scholar]
- Placke, T.; Kloepsch, R.; Dühnen, S.; Winter, M. Lithium ion, lithium metal, and alternative rechargeable battery technologies: The odyssey for high energy density. J. Solid State Electrochem. 2017, 21, 1939–1964. [Google Scholar] [CrossRef]
- Atzori, L.; Iera, A.; Morabito, G. The internet of things: A survey. Comput. Netw. 2010, 54, 2787–2805. [Google Scholar] [CrossRef]
- Liu, C.; Yang, C.; Zhang, X.; Chen, J. External integrity verification for outsourced big data in cloud and IoT: A big picture. Future Gener. Comput. Syst. 2015, 49, 58–67. [Google Scholar] [CrossRef]
- Song, T.; Li, R.; Mei, B.; Yu, J.; Xing, X.; Cheng, X. A privacy preserving communication protocol for IoT applications in smart homes. In Proceedings of the International Conference on Identification, Information and Knowledge in the Internet of Things (IIKI), Beijing, China, 20–21 October 2016; pp. 519–524. [Google Scholar]
- Zhang, Z.K.; Cho, M.C.Y.; Wang, C.W.; Hsu, C.W.; Chen, C.K.; Shieh, S. IoT security: Ongoing challenges and research opportunities. In Proceedings of the 7th IEEE International Conference on Service-Oriented Computing and Applications (SOCA), Matsue, Japan, 17–19 November 2014; pp. 230–234. [Google Scholar]
- Ciriani, V.; di Vimercati, S.D.C.; Foresti, S.; Samarati, P. k-Anonymous Data Mining: A Survey. In Privacy-Preserving Data Mining: Models and Algorithms; Springer: Boston, MA, USA, 2008; pp. 105–136. [Google Scholar]
- El Emam, K.; Dankar, F.K. Protecting Privacy Using k-Anonymity. J. Am. Med. Inform. Assoc. 2008, 15, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Machanavajjhala, A.; Kifer, D.; Gehrke, J.; Venkitasubramaniam, M. L-diversity: Privacy Beyond K-anonymity. ACM Trans. Knowl. Discov. Data 2007, 1. [Google Scholar] [CrossRef]
- Dwork, C. Differential Privacy. Automata, Languages and Programming; Bugliesi, M., Preneel, B., Sassone, V., Wegener, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–12. [Google Scholar]
- Microsoft Corporation. Differential Privacy for Everyone. Available online: download.microsoft.com/download/D/1/.../Differential_Privacy_for_Everyone.pdf (accessed on 1 November 2018).
- Dwork, C. Differential Privacy: A Survey of Results. Theory and Applications of Models of Computation; Agrawal, M., Du, D., Duan, Z., Li, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–19. [Google Scholar]
- Lee, J.; Clifton, C. How Much is Enough? Choosing ϵ for Differential Privacy. In Information Security; Lai, X., Zhou, J., Li, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 325–340. [Google Scholar]
- Hsu, J.; Gaboardi, M.; Haeberlen, A.; Khanna, S.; Narayan, A.; Pierce, B.C.; Roth, A. Differential Privacy: An Economic Method for Choosing Epsilon. In Proceedings of the 27th IEEE Computer Security Foundations Symposium, Vienna, Austria, 19–22 July 2014; pp. 398–410. [Google Scholar]
- Clifton, C.; Tassa, T. On syntactic anonymity and differential privacy. In Proceedings of the 29th IEEE International Conference on Data Engineering Workshops (ICDEW), Brisbane, Australia, 8–12 April 2013; pp. 88–93. [Google Scholar]
- Dankar, F.K.; El Emam, K. Practicing Differential Privacy in Health Care: A Review. Trans. Data Priv. 2013, 6, 35–67. [Google Scholar]
- Johnson, N.; Near, J.P.; Song, D. Towards Practical Differential Privacy for SQL Queries. Proc. VLDB Endow. 2018, 11, 526–539. [Google Scholar]
- Angeletti, F.; Chatzigiannakis, I.; Vitaletti, A. Privacy preserving data management in recruiting participants for digital clinical trials. In Proceedings of the First International Workshop on Human-Centered Sensing, Networking, and Systems, Delft, The Netherlands, 5 November 2017; pp. 7–12. [Google Scholar]
- Angeletti, F.; Chatzigiannakis, I.; Vitaletti, A. The role of blockchain and IoT in recruiting participants for digital clinical trials. In Proceedings of the 25th International Conference on Software, Telecommunications and Computer Networks (SoftCOM), Split, Croatia, 21–23 September 2017; pp. 1–5. [Google Scholar]
- Coulson, G.; Porter, B.; Chatzigiannakis, I.; Koninis, C.; Fischer, S.; Pfisterer, D.; Bimschas, D.; Braun, T.; Hurni, P.; Anwander, M.; et al. Flexible experimentation in wireless sensor networks. Commun. ACM 2012, 55, 82–90. [Google Scholar] [CrossRef]
- Sanchez, L.; Muñoz, L.; Galache, J.A.; Sotres, P.; Santana, J.R.; Gutierrez, V.; Ramdhany, R.; Gluhak, A.; Krco, S.; Theodoridis, E.; et al. SmartSantander: IoT experimentation over a smart city testbed. Comput. Netw. 2014, 61, 217–238. [Google Scholar] [CrossRef] [Green Version]
- Certicom Research: SEC 2—Recommended Elliptic Curve Domain Parameters. Available online: http://www.secg.org/sec2-v2.pdf (accessed on 1 November 2018).
- Biswas, A.S.; Bubna, A.; Doss, D.; Scheffler, S. Privacy Preserving K-Means Clustering; Technical Report; Massachusetts Institute of Technology: Cambridge, MA, USA, 2016. [Google Scholar]
- Samet, S.; Miri, A.; Orozco-Barbosa, L. Privacy Preserving k-Means Clustering in Multi-Party Environment; SECRYPT. INSTICC Press: Setubal, Portugal, 2007; pp. 381–385. [Google Scholar]
- Lichman, M. UCI Machine Learning Repository. Available online: https://archive.ics.uci.edu/ml/index.php (accessed on 1 November 2018).
- Saini, I.; Singh, D.; Khosla, A. QRS detection using K-Nearest Neighbor algorithm (KNN) and evaluation on standard ECG databases. J. Adv. Res. 2013, 4, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Moody, G.B.; Mark, R.G. The impact of the MIT-BIH arrhythmia database. IEEE Eng. Med. Biol. Mag. 2001, 20, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Onoue, T.; Goto, M.; Kobayashi, T.; Tominaga, T.; Ando, M.; Honda, H.; Yoshida, Y.; Tosaki, T.; Yokoi, H.; Kato, S.; et al. Randomized controlled trial for assessment of Internet of Things system to guide intensive glucose control in diabetes outpatients: Nagoya Health Navigator Study protocol. Nagoya J. Med. Sci. 2017, 79, 323. [Google Scholar] [PubMed]
- Han, K.H.; Bae, W.S. Proposing and verifying a security-enhanced protocol for IoT-based communication for medical devices. Clust. Comput. 2016, 19, 2335–2341. [Google Scholar] [CrossRef]
- Dimitrov, D.V. Medical internet of things and big data in healthcare. Healthc. Inform. Res. 2016, 22, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Amaxilatis, D.; Chatzigiannakis, I.; Mavrommati, I.; Vasileiou, E.; Vitaletti, A. Delivering elder-care environments utilizing TV-channel based mechanisms. JAISE 2017, 9, 783–798. [Google Scholar] [CrossRef]
- Sicari, S.; Rizzardi, A.; Grieco, L.; Piro, G.; Coen-Porisini, A. A policy enforcement framework for Internet of Things applications in the smart health. Smart Health 2017, 3, 39–74. [Google Scholar] [CrossRef]
- Shae, Z.; Tsai, J.J. On the design of a blockchain platform for clinical trial and precision medicine. In Proceedings of the 37th IEEE International Conference on Distributed Computing Systems (ICDCS), Atlanta, GA, USA, 5–8 June 2017; pp. 1972–1980. [Google Scholar]
- Whitaker, C.; Stevelink, S.; Fear, N. The use of Facebook in recruiting participants for health research purposes: A systematic review. J. Med. Internet Res. 2017, 19, e290. [Google Scholar] [CrossRef] [PubMed]
- Reuter, K.; Ukpolo, F.; Ward, E.; Wilson, M.L.; Angyan, P. Trial Promoter: A Web-Based Tool for Boosting the Promotion of Clinical Research through Social Media. J. Med. Internet Res. 2016, 18, e144. [Google Scholar] [CrossRef] [PubMed]
- Schwinn, T.; Hopkins, J.; Schinke, S.P.; Liu, X. Using Facebook ads with traditional paper mailings to recruit adolescent girls for a clinical trial. Addict. Behav. 2017, 65, 207–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melikyan, Z.A.; Greenia, D.E.; Corrada, M.M.; Hester, M.M.; Kawas, C.H.; Grill, J.D. Recruiting the Oldest-old for Clinical Research. Alzheimer Dis. Assoc. Disord. 2018. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Calfas, K.J.; Marshall, S.J.; Robinson, T.N.; Rock, C.L.; Huang, J.S.; Epstein-Corbin, M.; Servetas, C.; Donohue, M.C.; Norman, G.J.; et al. Clinical trial management of participant recruitment, enrollment, engagement, and retention in the SMART study using a Marketing and Information Technology (MARKIT) model. Contemp. Clin. Trials 2015, 42, 185–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiremath, S.; Yang, G.; Mankodiya, K. Wearable Internet of Things: Concept, architectural components and promises for person-centered healthcare. In Proceedings of the 4th EAI International Conference on Wireless Mobile Communication and Healthcare (Mobihealth), Athens, Greece, 3–5 November 2014; pp. 304–307. [Google Scholar]
- Patrick, F.; Young, A.H.; Williams, S.C.; Perkins, A.M. Prescreening clinical trial volunteers using an online personality questionnaire. Neuropsychiatr. Dis. Treat. 2018, 14, 2297. [Google Scholar] [CrossRef] [PubMed]
- Al-Majeed, S.S.; Al-Mejibli, I.S.; Karam, J. Home telehealth by internet of things (IoT). In Proceedings of the 28th IEEE Canadian Conference on Electrical and Computer Engineering (CCECE), Halifax, NS, USA, 3–6 May 2015; pp. 609–613. [Google Scholar]
- Tehrani, N.; Jin, Y. How Advances in the Internet of Things (IoT) Devices and Wearable Technology Will Impact the Pharmaceutical Industry. Res. Anal. J. 2018, 4, 1530–1533. [Google Scholar]
- Chatzigiannakis, I.; Strikos, A. A decentralized intrusion detection system for increasing security of wireless sensor networks. In Proceedings of the IEEE Conference on Emerging Technologies and Factory Automation, Patras, Greece, 25–28 September 2007; pp. 1408–1411. [Google Scholar]
- Chatzigiannakis, I.; Pyrgelis, A.; Spirakis, P.G.; Stamatiou, Y.C. Elliptic curve based zero knowledge proofs and their applicability on resource constrained devices. In Proceedings of the 8th IEEE International Conference on Mobile Adhoc and Sensor Systems (MASS), Valencia, Spain, 17–22 October 2011; pp. 715–720. [Google Scholar]
- Chatzigiannakis, I.; Konstantinou, E.; Liagkou, V.; Spirakis, P. Design, analysis and performance evaluation of group key establishment in wireless sensor networks. Electron. Notes Theor. Comput. Sci. 2007, 171, 17–31. [Google Scholar] [CrossRef]
- Chatzigiannakis, I.; Vitaletti, A.; Pyrgelis, A. A privacy-preserving smart parking system using an IoT elliptic curve based security platform. Comput. Commun. 2016, 89, 165–177. [Google Scholar] [CrossRef]
- Kerschbaum, F. Privacy-Preserving Computation. Privacy Technologies and Policy; Preneel, B., Ikonomou, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 41–54. [Google Scholar]
- Vu, D.; Slavkovic, A. Differential Privacy for Clinical Trial Data: Preliminary Evaluations. In Proceedings of the IEEE International Conference on Data Mining Workshops, Miami, FL, USA, 6 December 2009; pp. 138–143. [Google Scholar]
- Kenthapadi, K.; Korolova, A.; Mironov, I.; Mishra, N. Privacy via the Johnson-Lindenstrauss Transform. arXiv, 2012; arXiv:1204.2606. [Google Scholar]
- Xie, H.; Li, J.; Zhang, Q.; Wang, Y. Comparison among dimensionality reduction techniques based on Random Projection for cancer classification. Comput. Biol. Chem. 2016, 65, 165–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Kargupta, H.; Ryan, J. Random projection-based multiplicative data perturbation for privacy preserving distributed data mining. IEEE Trans. Knowl. Data Eng. 2006, 18, 92–106. [Google Scholar]
- Bianchi, T.; Bioglio, V.; Magli, E. Analysis of one-time random projections for privacy preserving compressed sensing. IEEE Trans. Inf. Forensics Secur. 2016, 11, 313–327. [Google Scholar] [CrossRef]
| Name | Age | ZIP | Age | ZIP | Disease | Age | ZIP | Disease |
|---|---|---|---|---|---|---|---|---|
| Joe | 15 | 1 | 15 | 1 | A | [15,18] | [1,2] | A |
| Nic | 18 | 2 | 18 | 2 | B | [15,18] | [1,2] | B |
| Lou | 35 | 3 | 35 | 3 | C | [35,40] | [3,4] | C |
| Mary | 40 | 4 | 40 | 4 | D | [35,40] | [3,4] | D |
| Parameter | Value |
|---|---|
| r | |
| x | |
| y |
| # | Dataset Name | Number of Samples | Number of Features |
|---|---|---|---|
| 1 | Arcene | 100 | 10,000 |
| 2 | EEG Eye State | 13,444 | 14 |
| 3 | Heart Disease | 270 | 13 |
| 4 | Gisette | 6000 | 5000 |
| PC | Gateway | |
|---|---|---|
| Filtering | 0.114648 s | 1.483026 s |
| FFT and PSD | 0.621372 s | 4.410873 s |
| Statistics | 0.016048 s | 0.115040 s |
| Total | 0.752068 s | 6.008939 s |
| # | PC | Gateway | |
|---|---|---|---|
| Support Vector Machines | 1 | 0.147322 s | 0.695988 s |
| 2 | 45.561 s | 565.288 s | |
| 3 | 0.005111 s | 0.061623 s | |
| 4 | 250.205 s | 1180.824 s | |
| Logistic Regression | 1 | 0.133114 s | 1.639809 s |
| 2 | 0.177369 s | 2.124157 s | |
| 3 | 0.001498 s | 0.020984 s | |
| 4 | 1.707945 s | 22.940 s | |
| k Nearest Neighbors | 1 | 0.010120 s | 0.086751 s |
| 2 | 0.009555 s | 0.125092 s | |
| 3 | 0.000355 s | 0.002438 s | |
| 4 | 1.315992 s | 18.851 s | |
| Gaussian Mixture Models | 1 | 0.245765 s | 2.103226 s |
| 2 | 0.667314 s | 7.934804 s | |
| 3 | 0.011209 s | 0.087351 s | |
| 4 | 30.658 s | Memory Error | |
| k-Means | 1 | 0.116964 s | 0.859489 s |
| 2 | 0.029887 s | 0.293893 s | |
| 3 | 0.003146 s | 0.035679 s | |
| 4 | 8.616173 s | Memory Error | |
| PCA | 1 | 0.463008 s | 3.250940 s |
| 2 | 0.055881 s | 0.662027 s | |
| 3 | 0.000539 s | 0.003660 s | |
| 4 | 40.488 s | Memory Error |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angeletti, F.; Chatzigiannakis, I.; Vitaletti, A. Towards an Architecture to Guarantee Both Data Privacy and Utility in the First Phases of Digital Clinical Trials. Sensors 2018, 18, 4175. https://doi.org/10.3390/s18124175
Angeletti F, Chatzigiannakis I, Vitaletti A. Towards an Architecture to Guarantee Both Data Privacy and Utility in the First Phases of Digital Clinical Trials. Sensors. 2018; 18(12):4175. https://doi.org/10.3390/s18124175
Chicago/Turabian StyleAngeletti, Fabio, Ioannis Chatzigiannakis, and Andrea Vitaletti. 2018. "Towards an Architecture to Guarantee Both Data Privacy and Utility in the First Phases of Digital Clinical Trials" Sensors 18, no. 12: 4175. https://doi.org/10.3390/s18124175







