A Facile Electrochemical Sensor Based on PyTS–CNTs for Simultaneous Determination of Cadmium and Lead Ions
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Materials
2.2. Instruments
2.3. Preparation of PyTS–CNTs
2.4. Preparation of PyTS–CNT-Modified PGE
2.5. DPASV Analysis
3. Results and Discussion
3.1. PyTS–CNT Characterization
3.2. Electrochemical Behaviors of Various Modified Electrodes
3.3. Optimization of Experimental Conditions
3.4. Analyses for Determination of Cd(II) and Pb(II)
3.5. Interferences
3.6. Repeatability and Reproducibility
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Srivastava, S.; Goyal, P. Heavy Metals: Environmental Threat. Environ. Sci. Eng. 2010, 1–10. [Google Scholar] [CrossRef]
- María-Hormigos, R.; Gismera, M.J.; Procopio, J.R.; Sevilla, M.T. Disposable screen-printed electrode modified with bismuth–PSS composites as high sensitive sensor for cadmium and lead determination. J. Electroanal. Chem. 2016, 767, 114–122. [Google Scholar] [CrossRef]
- Gao, F.; Gao, N.; Nishitani, A.; Tanaka, H. Rod-like hydroxyapatite and Nafion nanocomposite as an electrochemical matrix for simultaneous and sensitive detection of Hg2+, Cu2+, Pb2+ and Cd2+. J. Electroanal. Chem. 2016, 775, 212–218. [Google Scholar] [CrossRef]
- Maleki, A.; Pajootan, E.; Hayati, B. Ethyl acrylate grafted chitosan for heavy metal removal from wastewater: Equilibrium, kinetic and thermodynamic studies. J. Taiwan Inst. Chem. E 2015, 51, 127–134. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Garrido-Rodríguez, B.; Cutillas-Barreiro, L.; Araújo-Nespereira, P.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Influence of mussel shell on As and Cr competitive and non-competitive sorption–desorption kinetics in a mine soil: stirred flow chamber experiments. Geoderma 2014, 232–234, 300–308. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmed, S.; Swami, B.L.; Ikram, S. Adsorption of heavy metal ions: role of chitosan and cellulose for water treatment. Int. J. Pharmacogn. 2015, 2, 280–289. [Google Scholar]
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of heavy metals from industrial wastewaters: A review. ChemBioEng Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- Wang, T.; Yue, W. Carbon Nanotubes Heavy Metal Detection with Stripping Voltammetry: A Review Paper. Electroanalysis 2017, 29, 1–30. [Google Scholar] [CrossRef]
- Hamouz, O.C.S.A.; Estatie, M.K.; Morsy, M.A.; Saleh, T.A. Removal of cadmium ions from wastewater by dithiocarbamate functionalized pyrrole based terpolymers, Separation and Purification Technology. J. Taiwan Inst. Chem. E 2016, 70, 345–351. [Google Scholar] [CrossRef]
- Dai, H.; Wang, N.; Wang, D.; Ma, H.; Lin, M. An electrochemical sensor based on phytic acid functionalized polypyrrole/graphene oxide nanocomposites for simultaneous determination of Cd(II) and Pb(II). Chem. Eng. J. 2016, 299, 150–155. [Google Scholar] [CrossRef]
- Huang, H.; Zhu, W.; Gao, X.; Liu, X.; Ma, H. Synthesis of a novel electrode material containing phytic acid-polyaniline nanofibers for simultaneous determination of cadmium and lead ions. Anal. Chim. Acta 2016, 947, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Tomović, V.; Jokanović, M.; Tomović, M.; Lazović, M.; Šojić, B.; Škaljac, S.; Ivić, M.; Kocićtanackov, S.; Tomašević, I.; Martinović, A. Cadmium in liver and kidneys of domestic Balkan and Alpine dairy goat breeds from Montenegro and Serbia. Food Addit. Contam. 2017, 10, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Milne, A.; Landing, W.; Bizimis, M.; Morton, P. Determination of Mn, Fe, Co, Ni, Cu, Zn, Cd and Pb in seawater using high resolution magnetic sector inductively coupled mass spectrometry (HR-ICP-MS). Anal. Chim. Acta 2010, 665, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.C.; Costa, L.M.; Barbeira, P.J. Methyl oleate as matrix simulacrum for the simultaneous determination of metals in biodiesel samples by flame atomic emission spectroscopy. Talanta 2015, 138, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Fu, J.; Zheng, L.; Zhang, Z.; Xu, Y.; Zhu, X.; Zhu, D. Effects of organoamine template and transition metal coordination mode on the self-assembly of reduced polyoxomolybdenum phosphate. Crystengcomm 2011, 13, 5133–5141. [Google Scholar] [CrossRef]
- Guo, X.; Yun, Y.; Shanov, V.N.; Halsall, H.B.; Heineman, W.R. 1Carbon nanotubes heavy metal detection with strippingvoltammetry: A review paper. Electroanalysis 2011, 23, 1252–1259. [Google Scholar] [CrossRef]
- Lee, S.; Oh, J.; Kim, D.; Piao, Y. A sensitive electrochemical sensor using an iron oxide/graphene composite for the simultaneous detection of heavy metal ions. Talanta 2016, 160, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.; García, M.B.G.; Santos, D.H.; Fanjul-Bolado, P.; Ribotti, A.; Mccaul, M.; Diamond, D.; Magni, P. Screen-printed electrodes for environmental monitoring of heavy metal ions: A review. Microchim. Acta 2016, 183, 503–517. [Google Scholar] [CrossRef]
- Huang, H.; Chen, T.; Liu, X.; Ma, H. Ultrasensitive and simultaneous detection of heavy metal ions based on three-dimensional graphene-carbon nanotubes hybrid electrode materials. Anal. Chim. Acta 2014, 852, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Stripping-based electrochemical metal sensors for environmental monitoring. Comprehen. Anal. Chem. 2007, 49, 131–141. [Google Scholar]
- Zhao, D.; Wang, T.; Han, D.; Rusinek, C.; Steckl, A.J.; Heineman, W.R. Electrospun Carbon Nanofiber Modified Electrodes for Stripping Voltammetry. Anal. Chem. 2015, 87, 9315–9321. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Xu, H.; Zhou, S.; Song, T.; Wang, H.; Li, S.; Gan, W.; Yuan, Q. Simultaneous detection of Cd(II) and Pb(II) by differential pulse anodic stripping voltammetry at a nitrogen-doped microporous carbon/Nafion/bismuth-film electrode. Electrochim. Acta 2014, 143, 143–151. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, F.; Tang, J. Sodium alginate decorated carbon nanotubes-graphene composite aerogel for heavy metal ions detection. Electrochemistry 2015, 83, 84–90. [Google Scholar] [CrossRef]
- Yuan, L.; Li, H.; Xu, X.; Zhang, J.; Wang, N.; Yu, H. Electrokinetic remediation of heavy metals contaminated kaolin by a CNT-covered polyethylene terephthalate yarn cathode. Electrochim. Acta 2016, 213, 140–147. [Google Scholar] [CrossRef]
- Zhu, S.; Xu, G. Single-walled carbon nanohorns and their applications. Nanoscale 2010, 2, 2538–2549. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, M.; Khanbolouki, P. Carbon Nanotubes: Synthesis, Characterization, and Applications. Proc. SPIE 2018, 240, 280–285. [Google Scholar]
- Robinson, J.E.; Heineman, W.R.; Sagle, L.B.; Meyyappan, M.; Koehne, J.E. Carbon nanofiber electrode array for the detection of lead. Electrochem. Commun. 2016, 73, 89–93. [Google Scholar] [CrossRef]
- Hayati, B.; Maleki, A.; Najafi, F.; Daraei, H.; Gharibi, F.; Mckay, G. Super high removal capacities of heavy metals (Pb2+ and Cu2+) using CNT dendrimer. J. Hazard. Mater. 2017, 336, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Hamza, A.; El-Refaei, S.; Elzatahry, A.; Abdullah, A. High Electrocatalytic Performance of CuCoNi@CNTs Modified Glassy Carbon Electrode towards Methanol Oxidation in Alkaline Medium. Appl. Sci. 2017, 7, 64. [Google Scholar] [CrossRef]
- Yang, H.; Hernandez, Y.; Schlierf, A.; Felten, A.; Eckmann, A.; Johal, S.; Louette, P.; Pireaux, J.J.; Feng, X.; Mullen, K. A simple method for graphene production based on exfoliation of graphite in water using 1-pyrenesulfonic acid sodium salt. Carbon 2013, 53, 357–365. [Google Scholar] [CrossRef]
- Fujigaya, T.; Nakashima, N. Non-covalent polymer wrapping of carbon nanotubes and the role of wrapped polymers as functional dispersants. Sci. Tech. Adv. Mat. 2015, 16, 024802. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Sulfur and Nitrogen Dual-Doped Mesoporous Graphene Electrocatalyst for Oxygen Reduction with Synergistically Enhanced Performance. Angew. Chem. 2012, 51, 11496–11500. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhou, Y.; Li, Z.; Ma, Y.; Pei, C. One step fabrication of Mn3O4/carbonated bacterial cellulose with excellent catalytic performance upon ammonium perchlorate decomposition. Mater. Res. Bull. 2014, 60, 802–807. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, D.; Zhao, L.; Lu, X.; Zhang, P.; He, S.; Hu, G.; Tang, X. Synthesis of a thiacalix[4]arenetetrasulfonate-functionalized reduced graphene oxide adsorbent for the removal of lead(II) and cadmium(II) from aqueous solutions. RSC Adv. 2016, 6, 113352–113365. [Google Scholar] [CrossRef]
- Joshi, A.; Nagaiah, T.C. Nitrogen-doped carbon nanotubes for sensitive and selective determination of heavy metals. RSC Adv. 2015, 5, 105119–105127. [Google Scholar] [CrossRef]
- Ashrafi, A.M.; Cerovac, S.; Mudrić, S.; Guzsvány, V.; Husáková, L.; Urbanová, I.; Vytřas, K. Antimony nanoparticle-multiwalled carbon nanotubes composite immobilized at carbon paste electrode for determination of trace heavy metals. Sensor Ctuat. B-Chem. 2014, 191, 320–325. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, H.; Liu, G.; Wang, Z.; Cheng, J. Simultaneous determination of trace Cd(II) and Pb(II) based on Bi/Nafion/reduced graphene oxide-gold nanoparticle nanocomposite film-modified glassy carbon electrode by one-step electrodeposition. Ionics 2016, 3, 767–777. [Google Scholar] [CrossRef]
- Cerovac, S.; Guzsvány, V.; Kónya, Z.; Ashrafi, A.M.; Rončević, S.; Kukovecz, Á.; Dalmacija, B.; Vytřas, K. Trace level voltammetric determination of lead and cadmium in sediment pore water by a bismuth-oxychloride particle-multiwalled carbon nanotube composite modified glassy carbon electrod. Talanta 2015, 134, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Madhu, R.; Sankar, K.V.; Chen, S.M.; Selvan, R.K. Eco-friendly synthesis of activated carbon from dead mango leaves for the ultrahigh sensitive detection of toxic heavy metal ions and energy storage applications. RSC Adv. 2013, 4, 1225–1233. [Google Scholar] [CrossRef]
- Saeed, A.A.; Singh, B.; Abbas, M.N.; Dempsey, E. Evaluation of Bismuth Modified Carbon Thread Electrode for Simultaneous and Highly Sensitive Cd(II) and Pb(II) Determination. Electroanalysis 2016, 28, 2205–2213. [Google Scholar] [CrossRef]
- Li, X.; Zhou, H.; Fu, C.; Wang, F.; Ding, Y.; Kuang, Y. A novel design of engineered multi-walled carbon nanotubes material and its improved performance in simultaneous detection of Cd(II) and Pb(II) by square wave anodic stripping voltammetry. Sensor Actuat. B-Chem. 2016, 236, 144–152. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, L.; Huang, B.; Jia, N.; Liang, T.; Yao, S. Simultaneous determination of Cd(II) and Pb(II) using square wave anodic stripping voltammetry at a gold nanoparticle-graphene-cysteine composite modified bismuth film electrode. Electrochim. Acta 2014, 115, 471–477. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, B.; Ji, L.; Wang, F.; Yuan, Q.; Hu, G.; Dong, A.; Gan, W. An efficient electrochemical sensor based on three-dimensionally interconnected mesoporous graphene framework for simultaneous determination of Cd(II) and Pb(II). Electrochim. Acta 2016, 222, 1371–1377. [Google Scholar] [CrossRef]
- Khieu, D.Q.; Son, B.H.D.; Chau, V.T.T.; Du, P.D.; Phong, N.H.; Chau, N.T.D. 3-mercaptopropyltrimethoxysilane modified diatomite: preparation and application for voltammetric determination of lead(II) and cadmium(II). J. Chem. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Nomura, C.; Scorreia, P.R.; Moliveira, P.; Voliveira, E. Nomura C S, Correia P R M, Oliveira P V, et al. W+Rh as permanent chemical modifier in simultaneous atomic absorption spectrometry: interference studies on As, Cd, Pb and Se determination. J. Brazil. Chem. Soc. 2004, 15, 75–82. [Google Scholar] [CrossRef]

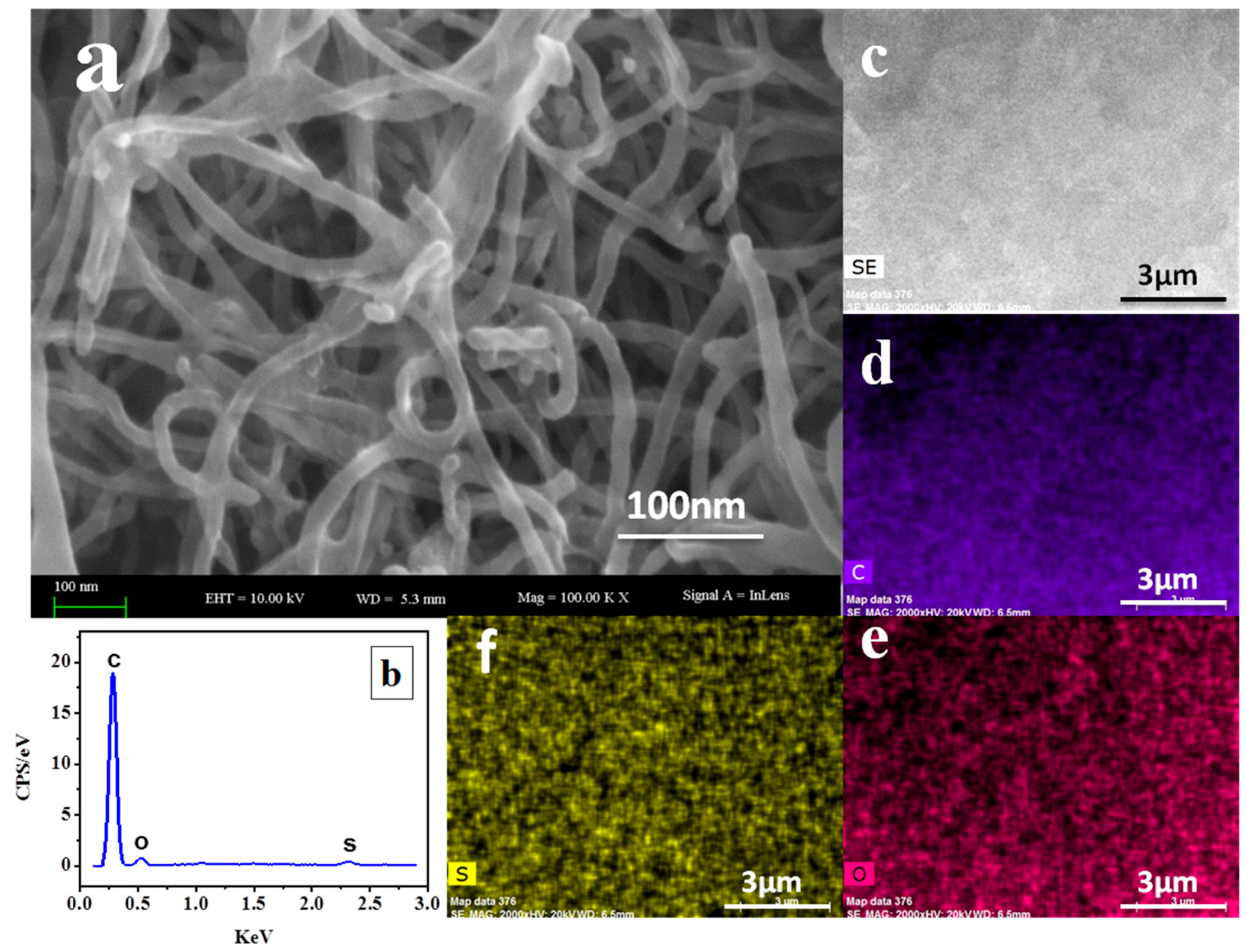
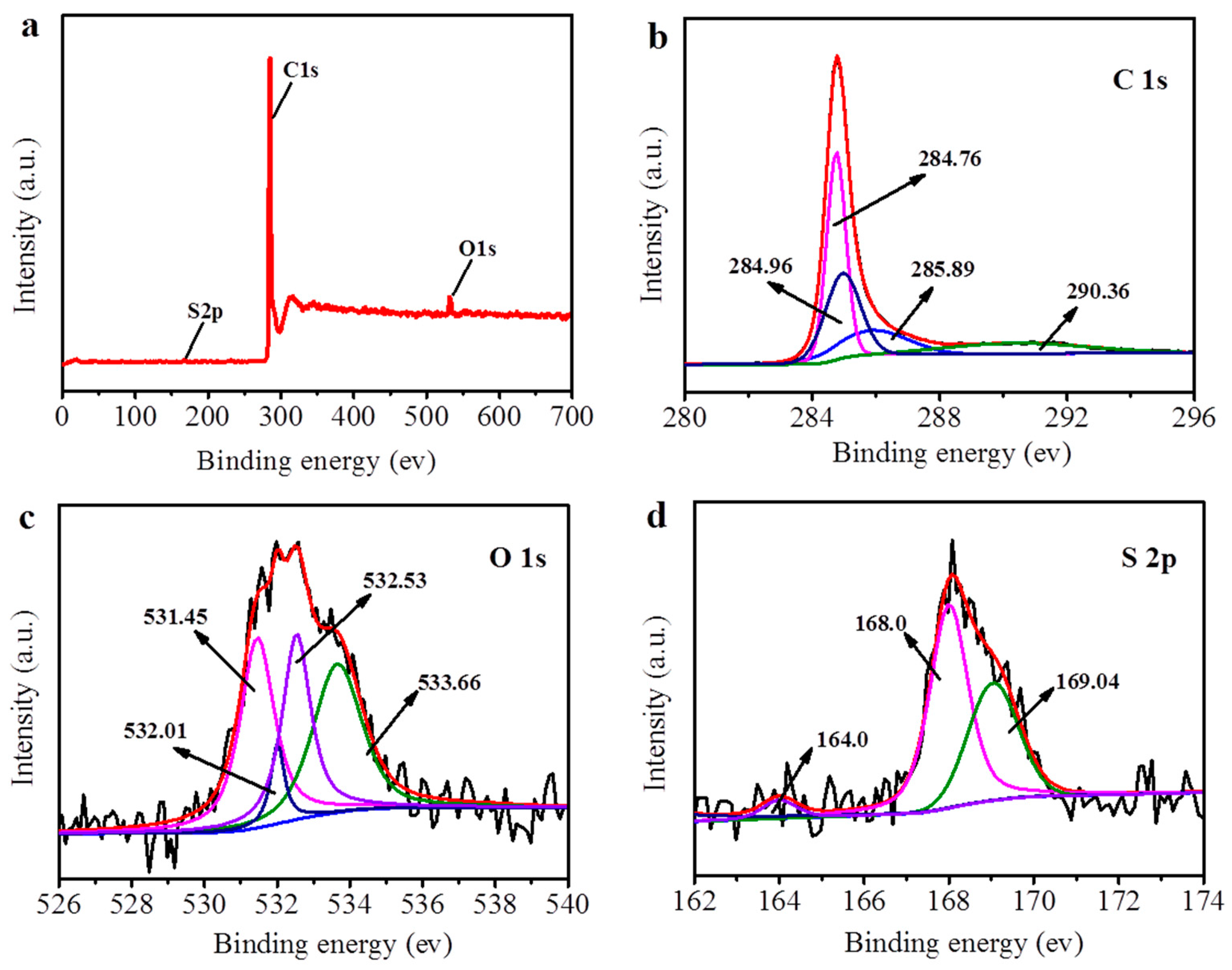


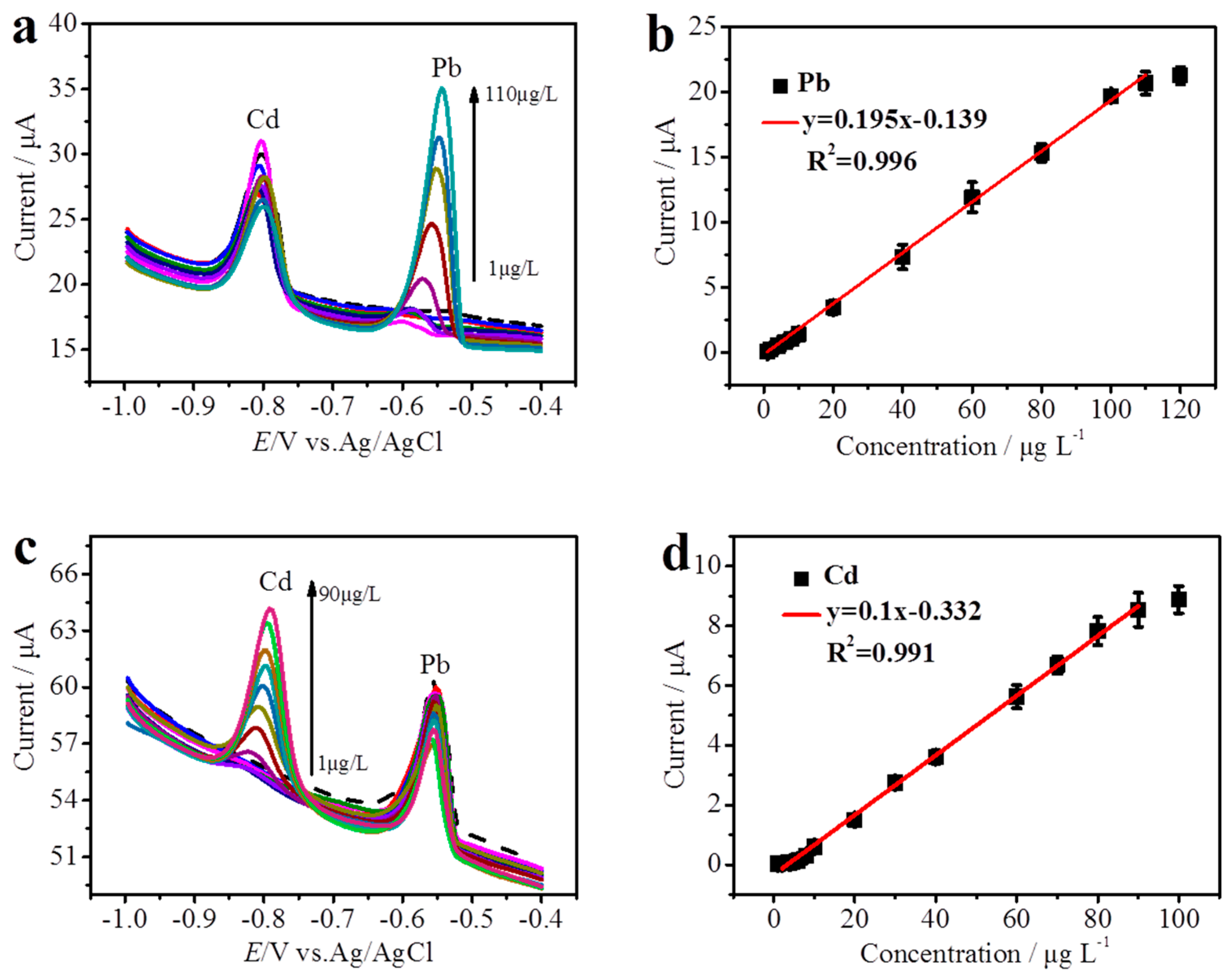
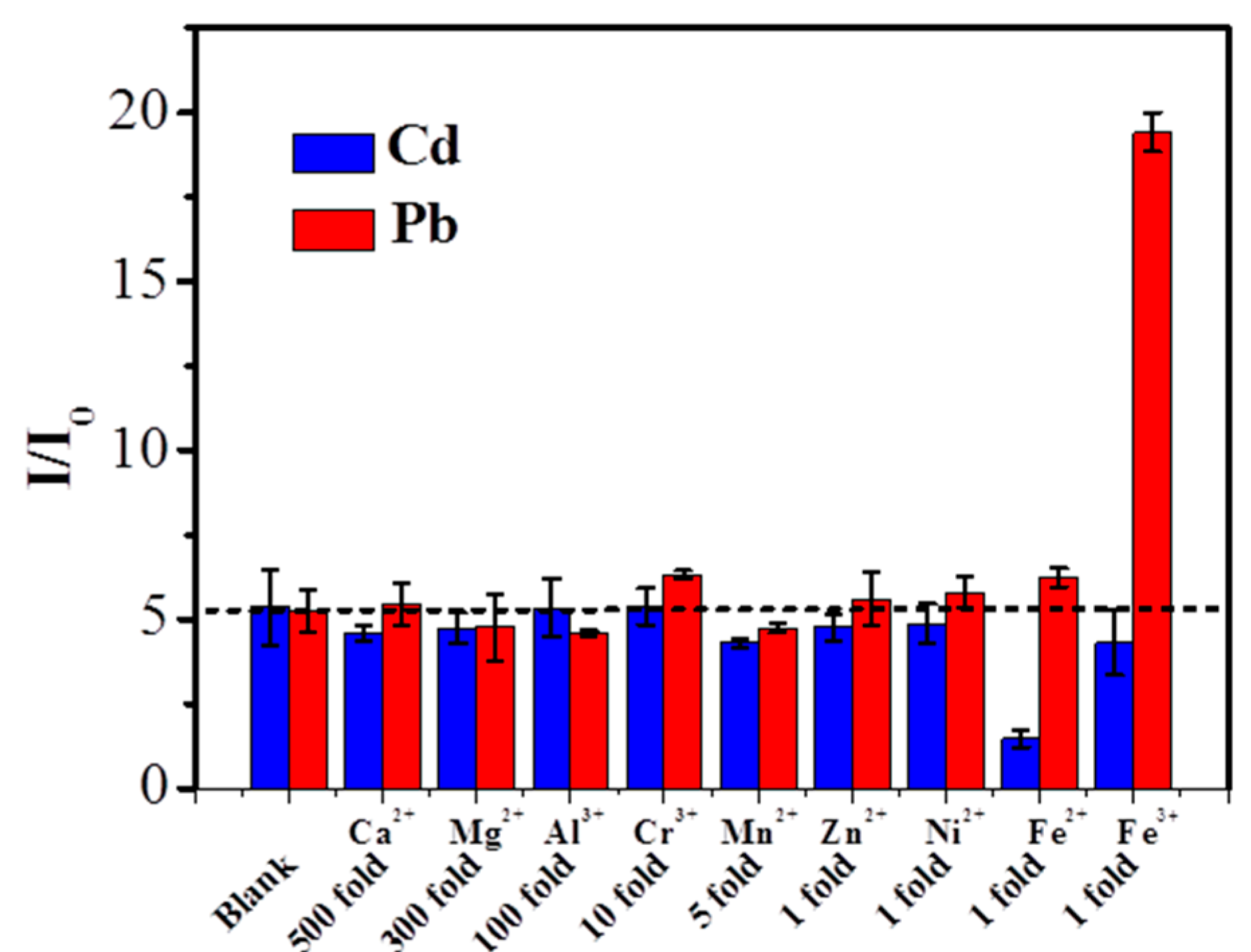
| Electrode Substrate | Method | Deposition Time (s) | Linear Range (μg L−1) | Limit of Detection (µg L−1) | Reference | ||
|---|---|---|---|---|---|---|---|
| Cd2+ | Pb2+ | Cd2+ | Pb2+ | ||||
| PA/PPy/GO/GCE | DPASV | 200 | 5–150 | 5–150 | 2.13 | 0.41 | [10] |
| G/Nafion/MWCNTs/Bi/GCE | DPASV | 180 | 0.5–30 | 0.5–30 | 0.1 | 0.2 | [19] |
| CNF/Nafion/GCE | OSWSV | 300 | - | - | 0.19 | 0.31 | [21] |
| N–CNT/GCE | SWASV | 11–11,200 | 2–1450 | 5.6 | 0.21 | [35] | |
| SbNP/MWCNT/CPE | DPASV | 120 | 10–60 | 10–60 | 0.8 | 0.7 | [36] |
| Bi/Nafion/RGO-GNPs/GCE | SWASV | 140 | 1–90 | 1–90 | 0.12 | 0.08 | [37] |
| BiOCl/MWCNT–GCE | SWASV | 120 | 5–50 | 5–50 | 1.2 | 0.6 | [38] |
| SNAC/GCE | DPASV | 10.1–539.6 | 18.6–1180.0 | 2.7 | 1.2 | [39] | |
| Bi/IPA-treated CTE | SWASV | 300 | 5–110 | 5–110 | 1.08 | 0.87 | [40] |
| MWCNTs/Bi–GCE | DPASV | 180 | 0.5–30 | 0.5–30 | 0.1 | 0.2 | [41] |
| Au–GN–Cys/GCE | SWASV | 800 | 0.4–40 | 0.5–40 | 0.1 | 0.05 | [42] |
| Bi/MGF–Nafion/GCE | DPASV | 360 | 2–70 | 0.5–110 | 0.5 | 0.1 | [43] |
| Diatomite–MPTMS/GCE | DPASV | 300 | 20–300 | 20–150 | 15.9 | 6.9 | [44] |
| PyTS–CNTs/Nafion/PGE | DPASV | 270 | 1–90 | 1–100 | 0.8 | 0.02 | This work |
| Analyte | 1 | 2 | 3 | 4 | 5 | Found (μg L−1) | RSD (%) | |
|---|---|---|---|---|---|---|---|---|
| Reproducibility n = 5 | Cd2+ | 4.582 | 5.201 | 4.431 | 4.886 | 4.607 | 4.741 ± 0.304 | 6.4 |
| Pb2+ | 6.421 | 6.902 | 6.175 | 6.474 | 6.674 | 6.529 ± 0.274 | 4.2 | |
| Repeatability n = 1 | Cd2+ | 4.713 | 4.469 | 4.734 | 4.5 | 4.717 | 4.627 ± 0.130 | 2.8 |
| Pb2+ | 6.83 | 6.831 | 6.805 | 6.458 | 6.807 | 6.746 ± 0.162 | 2.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, R.; Liu, N.; Gao, S.; Mamat, X.; Su, Y.; Wagberg, T.; Li, Y.; Hu, X.; Hu, G. A Facile Electrochemical Sensor Based on PyTS–CNTs for Simultaneous Determination of Cadmium and Lead Ions. Sensors 2018, 18, 1567. https://doi.org/10.3390/s18051567
Jiang R, Liu N, Gao S, Mamat X, Su Y, Wagberg T, Li Y, Hu X, Hu G. A Facile Electrochemical Sensor Based on PyTS–CNTs for Simultaneous Determination of Cadmium and Lead Ions. Sensors. 2018; 18(5):1567. https://doi.org/10.3390/s18051567
Chicago/Turabian StyleJiang, Ruyuan, Niantao Liu, Sanshuang Gao, Xamxikamar Mamat, Yuhong Su, Thomas Wagberg, Yongtao Li, Xun Hu, and Guangzhi Hu. 2018. "A Facile Electrochemical Sensor Based on PyTS–CNTs for Simultaneous Determination of Cadmium and Lead Ions" Sensors 18, no. 5: 1567. https://doi.org/10.3390/s18051567
APA StyleJiang, R., Liu, N., Gao, S., Mamat, X., Su, Y., Wagberg, T., Li, Y., Hu, X., & Hu, G. (2018). A Facile Electrochemical Sensor Based on PyTS–CNTs for Simultaneous Determination of Cadmium and Lead Ions. Sensors, 18(5), 1567. https://doi.org/10.3390/s18051567






