Multifunctional Freestanding Microprobes for Potential Biological Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Stainless-Steel Substrate
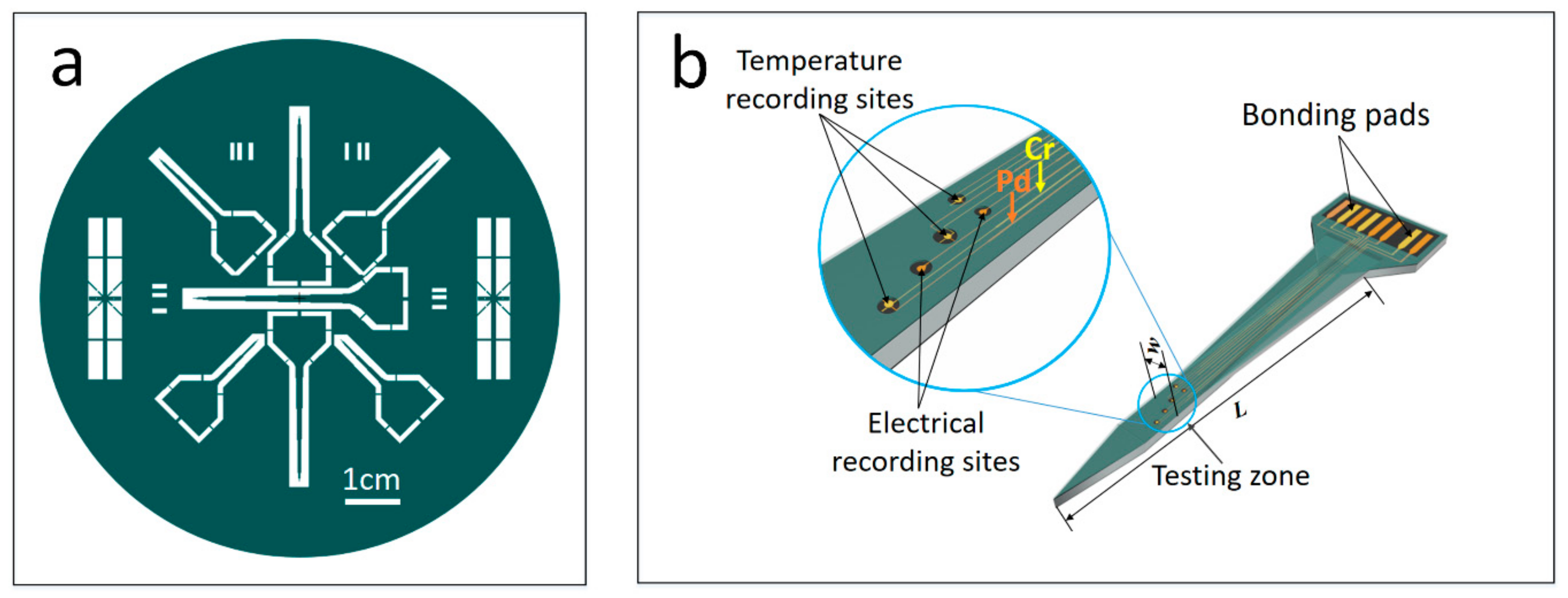
2.2. Design of 3D Probes
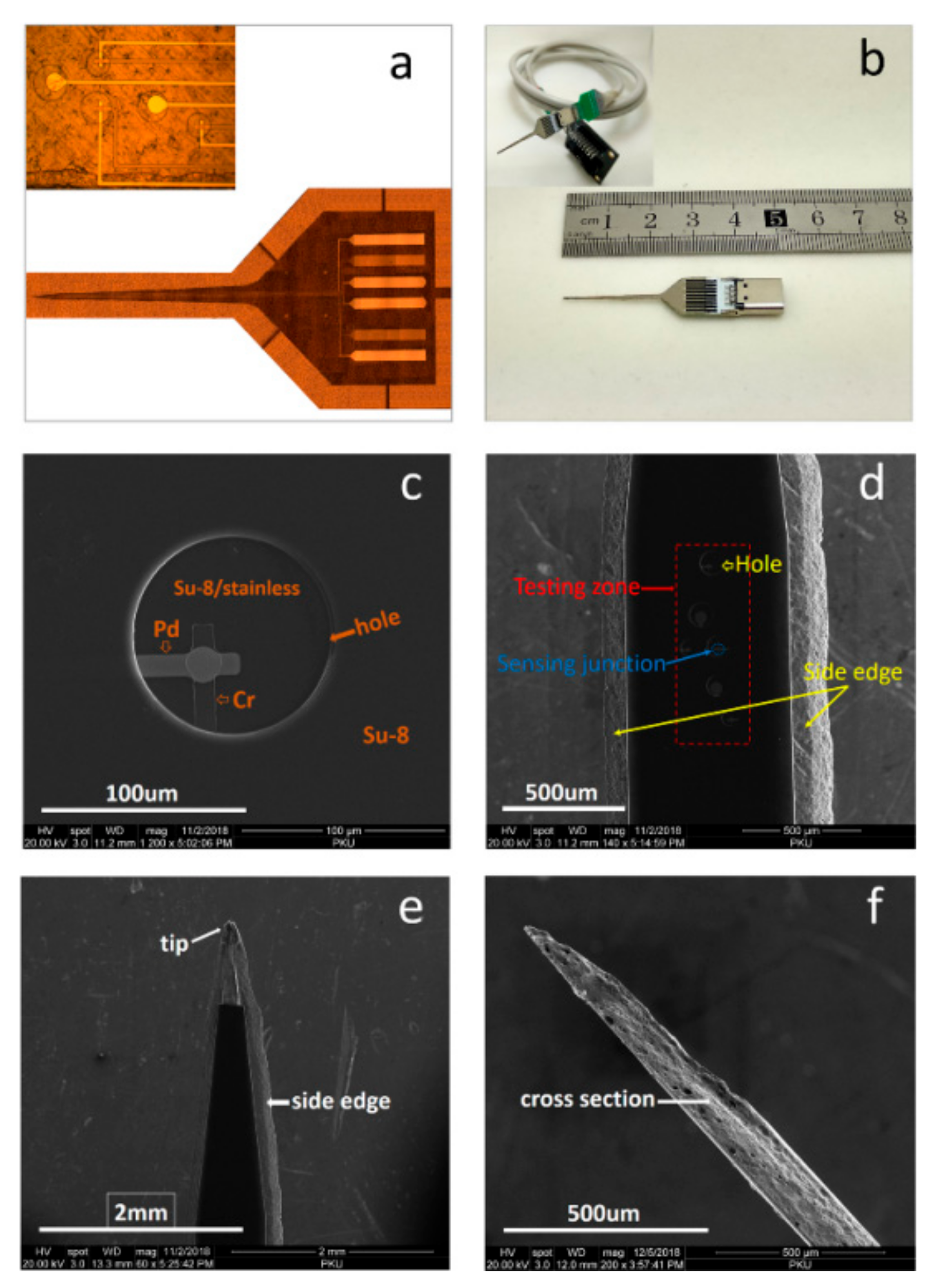
2.3. Fabrication of Freestanding Microprobes
2.4. Device Testing
3. Results and Discussions
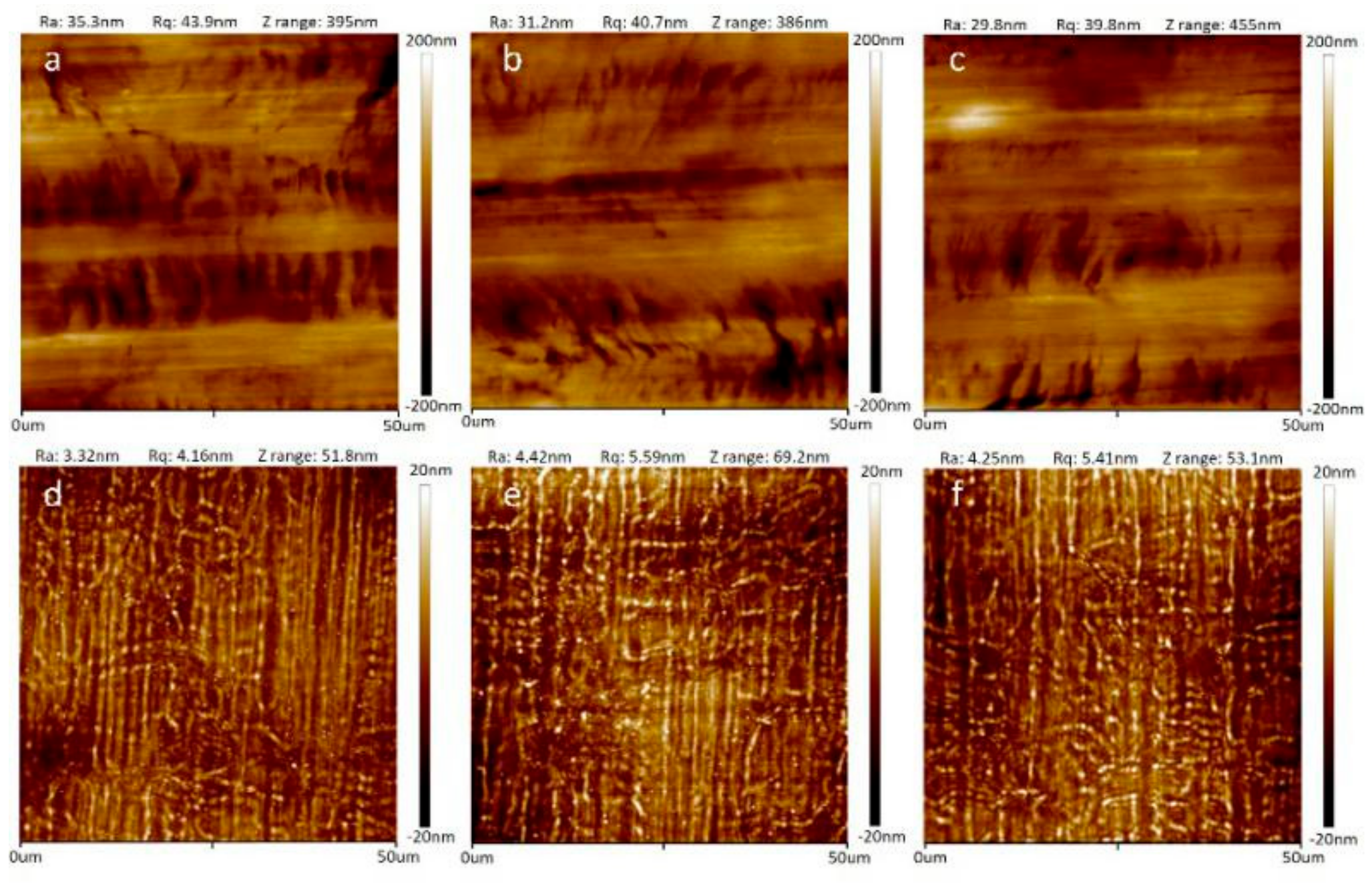
3.1. Improvement of Surface Smoothness after Coating SU-8
3.2. Lithography and Etching Results
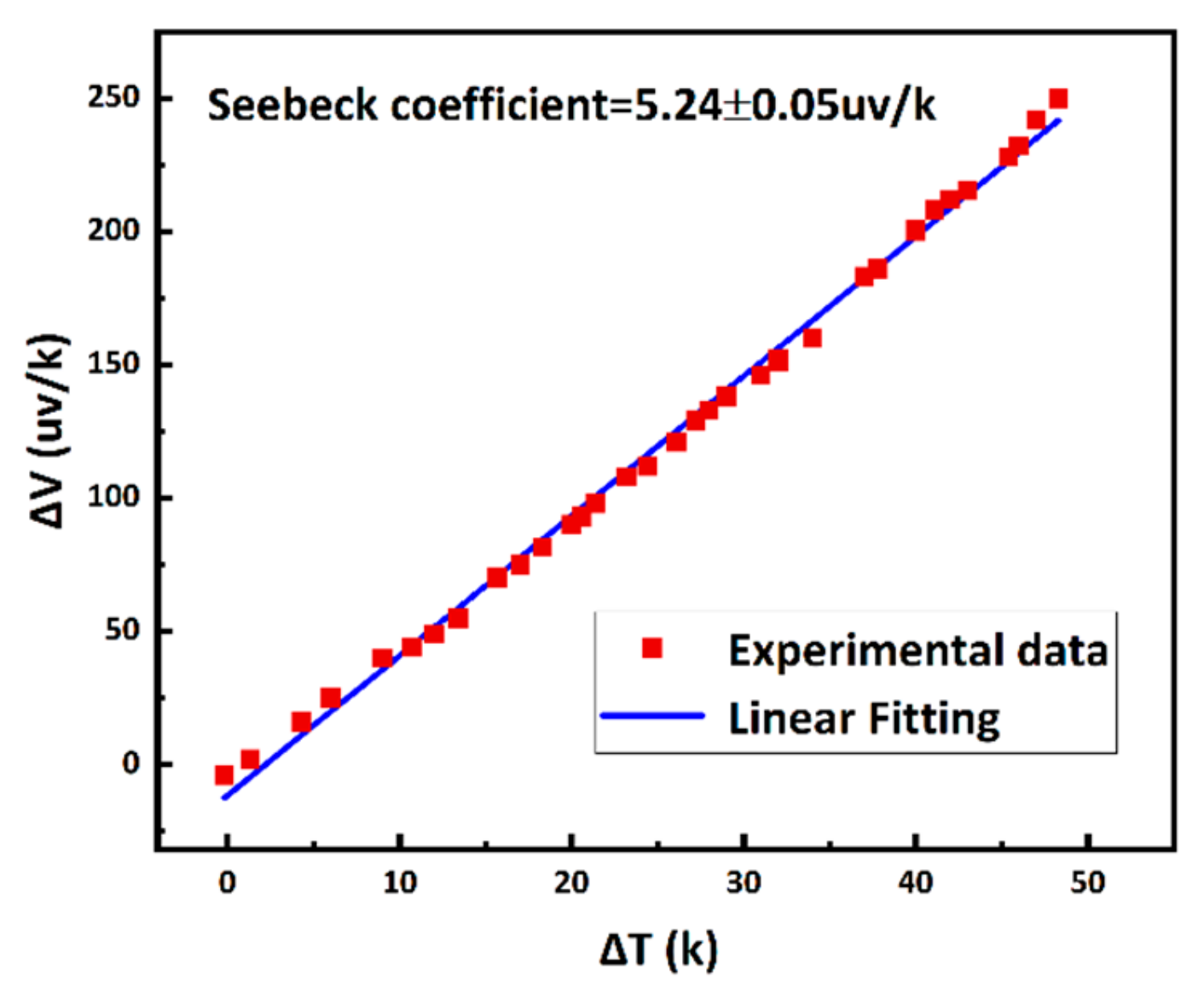
3.3. Sensor Calibration
3.4. A. C. Impedance
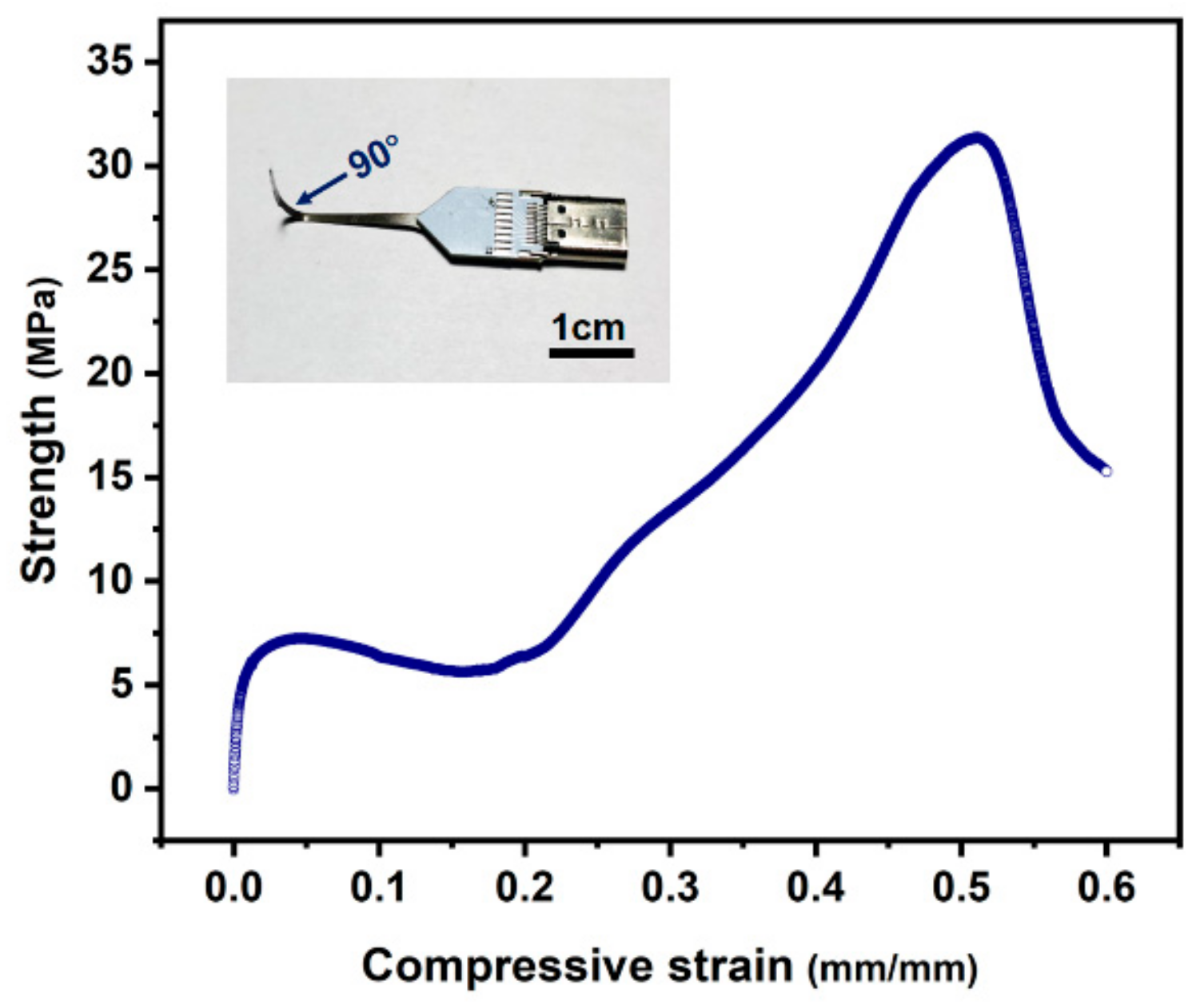
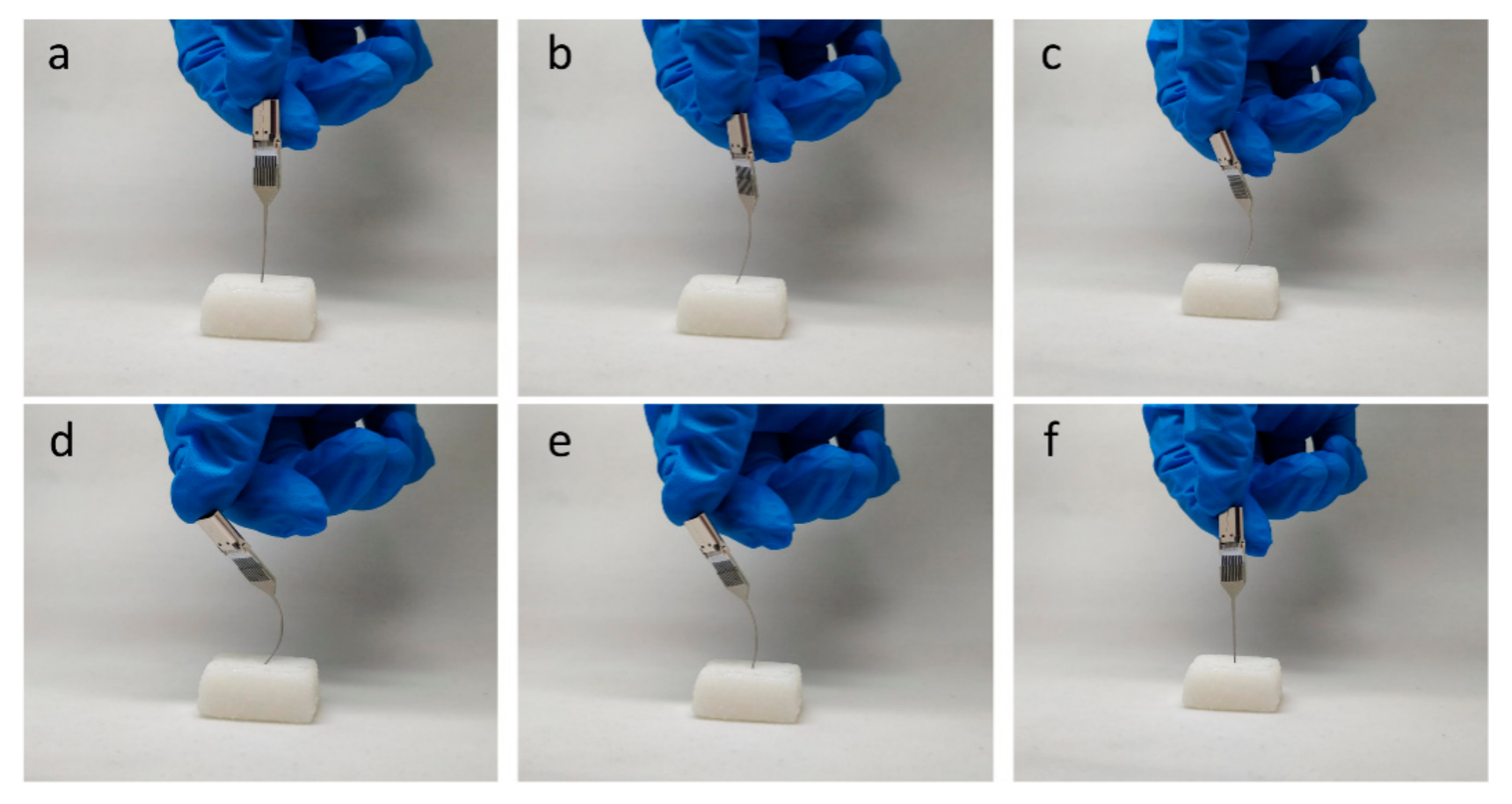
3.5. Testing of Mechanical Strength
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
Ethical Approval
References
- Kim, D.H.; Lu, N.; Ghaffari, R.; Kim, Y.S.; Lee, S.P.; Xu, L.; Wu, J.; Kim, R.H.; Song, J.; Liu, Z.; et al. Materials for multifunctional balloon catheters with capabilities in cardiac electrophysiological mapping and ablation therapy. Nat. Mater. 2011, 10, 316–323. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Viventi, J.; Amsden, J.J.; Xiao, J.; Vigeland, L.; Kim, Y.S.; Blanco, J.A.; Panilaitis, B.; Frechette, E.S.; Contreras, D.; et al. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat. Mater. 2010, 9, 511–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.H.; Ahn, J.H.; Choi, W.M.; Kim, H.S.; Kim, T.H.; Song, J.; Huang, Y.Y.; Liu, Z.; Lu, C.; Rogers, J.A. Stretchable and foldable silicon integrated circuits. Science 2008, 320, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Song, J.; Choi, W.M.; Kim, H.S.; Rogers, J.A. Materials and noncoplanar mesh designs for integrated circuits with linear elastic responses to extreme mechanical deformations. Proc. Nat. Acad. Sci. USA 2008, 105, 18675–18680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viventi, J.; Kim, D.H.; Vigeland, L.; Frechette, E.S.; Blanco, J.A.; Kim, Y.S.; Avrin, A.E.; Tiruvadi, V.R.; Hwang, S.W.; Vanleer, A.C.; et al. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat. Neurosci. 2011, 14, 1599–1605. [Google Scholar] [Green Version]
- Kim, D.H.; Ghaffari, R.; Lu, N.; Rogers, J.A. Flexible and stretchable electronics for biointegrated devices. Annu. Rev. Biomed. Eng. 2012, 14, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Cavazos Sepulveda, A.C.; Diaz Cordero, M.S.; Carreño, A.A.A.; Nassar, J.M.; Hussain, M.M. Stretchable and foldable silicon-based electronics. Appl. Phys. Lett. 2017, 110, 134103. [Google Scholar] [CrossRef]
- Chen, C.H.; Chuang, S.C.; Lee, Y.T.; Chang, Y.C.; Yeh, S.R.; Yao, D.J. Three-dimensional flexible microprobe for recording the neural signal. In Proceedings of the IEEE International Conference on Nano/Molecular Medicine & Engineering, Tainan, Taiwan, 18–21 October 2010. [Google Scholar]
- Chen, C.H.; Chuang, S.C.; Su, H.C.; Hsu, W.L.; Yew, T.R.; Chang, Y.C.; Yeh, S.R.; Yao, D.J. A three-dimensional flexible microprobe array for neural recording assembled through electrostatic actuation. Lab Chip 2011, 11, 1647–1655. [Google Scholar] [CrossRef]
- Tan, K.L.; Chen, W.; Damalerio, R.; Lim, R.; Chungpaiboonpatana, S.; Cheng, M.Y. Evaluation of biodegradable coating on the stiffness control of the polyimide-based probe used in neural devices. In Proceedings of the IEEE Electronics Packaging & Technology Conference, Singapore, 2–4 December 2015. [Google Scholar]
- Kuo, J.T.; Kim, B.J.; Hara, S.A.; Lee, C.D.; Gutierrez, C.A.; Hoang, T.Q.; Meng, E. Novel flexible Parylene neural probe with 3D sheath structure for enhancing tissue integration. Lab Chip 2013, 13, 554–561. [Google Scholar] [CrossRef]
- Kim, B.J.; Kuo, J.T.; Hara, S.A.; Lee, C.D.; Yu, L.; Gutierrez, C.A.; Hoang, T.Q.; Pikov, V.; Meng, E. 3D Parylene sheath neural probe for chronic recordings. J. Neural Eng. 2013, 10, 045002. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Lieber, C.M. Nanoelectronics meets biology: From new nanoscale devices for live-cell recording to 3D innervated tissues. Chem. Asian J. 2013, 8, 2304–2314. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Viveros, R.D.; Zwang, T.J.; Yang, X.; Lieber, C.M. Tissue-like neural probes for understanding and modulating the brain. Biochemistry 2018, 57, 3995–4004. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Hong, G.; Gao, T.; Lieber, C.M. Mesh nanoelectronics: Seamless integration of electronics with tissues. Acc. Chem. Res. 2018, 51, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Yang, X.; Zhou, T.; Lieber, C.M. Mesh electronics: A new paradigm for tissue-like brain probes. Curr. Opin. Neurobiol. 2018, 50, 33–41. [Google Scholar] [CrossRef]
- Duan, X.; Fu, T.M.; Liu, J.; Lieber, C.M. Nanoelectronics-biology frontier: From nanoscopic probes for action potential recording in live cells to three-dimensional cyborg tissues. Nano Today 2013, 8, 351–373. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.; Liu, J.; Fu, T.-M.; Dai, X.; Zhou, W.; Lieber, C.M. Three-dimensional macroporous nanoelectronic networks as minimally invasive brain probes. Nat. Mater. 2015, 14, 1286–1292. [Google Scholar] [CrossRef] [Green Version]
- Goßler, C.; Bierbrauer, C.; Moser, R.; Kunzer, M.; Holc, K.; Pletschen, W.; Köhler, K.; Wagner, J.; Schwaerzle, M.; Ruther, P.; et al. GaN-based micro-LED arrays on flexible substrates for optical cochlear implants. J. Phys. D Appl. Phys. 2014, 47, 205401. [Google Scholar] [CrossRef]
- Knisely, K.; Zhao, C.; Grosh, K. A MEMS AlN transducer array with flexible interconnections for use as a cochlear implant. AIP Conf. Proc. 2015, 1703, 100004. [Google Scholar]
- Rousche, P.J.; Pellinen, D.S.; Pivin, D.P.; Williams, J.C.; Vetter, R.J.; Kipke, D.R. Flexible polyimide-based intracortical electrode arrays with bioactive capability. IEEE Trans. Biomed. Eng. 2001, 48, 361–371. [Google Scholar] [CrossRef]
- Wise, K.D.; Angell, J.B.; Starr, A. An integrated-circuit approach to extracellular microelectrodes. IEEE Trans. Biomed. Eng. 1970, BME-17, 238–247. [Google Scholar] [CrossRef]
- Xu, C.; Lemon, W.; Liu, C. Design and fabrication of a high-density metal microelectrode array for neural recording. Sensor. Actuators A (Phys.) 2002, 96, 78–85. [Google Scholar] [CrossRef]
- Ensell, G.; Banks, D.J.; Ewins, D.J.; Balachandran, W.; Richards, P.R. Silicon-based microelectrodes for neurophysiology fabricated using a gold metallization/nitride passivation system. J. Microelectromech. Syst. 1996, 5, 117–121. [Google Scholar] [CrossRef]
- Anderson, D.J.; Najafi, K.; Tanghe, S.J.; Evans, D.A.; Levy, K.L.; Hetke, J.F.; Xue, X.L.; Zappia, J.J.; Wise, K.D. Batch-fabricated thin-film electrodes for stimulation of the central auditory system. IEEE Trans. Biomed. Eng. 1989, 36, 693–704. [Google Scholar] [CrossRef]
- Kovacs, G.T.; Storment, C.W.; Rosen, J.M. Regeneration microelectrode array for peripheral nerve recording and stimulation. IEEE Trans. Biomed. Eng. 1992, 39, 893–902. [Google Scholar] [CrossRef]
- Kipke, D.R.; Pellinen, D.S.; Vetter, R.J. Advanced neural implants using thin-film polymers. In Proceedings of the IEEE International Symposium on Circuits & Systems, Phoenix-Scottsdale, AZ, USA, 26–29 May 2002. [Google Scholar]
- Norlin, P.; Kindlundh, M.; Mouroux, A.; Yoshida, K.; Hofmann, U.G. A 32-site neural recording probe fabricated by DRIE of SOI substrates. J. Micromech. Microeng. 2002, 12, 414–419. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, H.A.; Frenk, S.; Lettvin, J.Y. Glass-coated tungsten microelectrodes. Science 1965, 148, 1462–1464. [Google Scholar] [CrossRef] [PubMed]
- Fomani, A.A.; Moradi, M.; Assaf, S.; Mansour, R.R. 3D Microprobes for deep brain stimulation and recording. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–3 September 2010. [Google Scholar]
- Motta, P.S.; Judy, J.W. Multielectrode microprobes for deep-brain stimulation fabricated with a customizable 3-D electroplating process. IEEE Trans. Biomed. Eng. 2005, 52, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Medioni, G.; Winstein, C.J.; Blanco, C. Home monitoring musculo-skeletal disorders with a single 3D sensor. In Proceedings of the 2013 IEEE Conference on Computer Vision and Pattern Recognition Workshops, Portland, OR, USA, 23–28 June 2013; pp. 521–528. [Google Scholar]
- Lee, S.; Ahn, J.H.; Seo, J.M.; Chung, H.; Cho, D.I. Electrical characterization of 3D Au microelectrodes for use in retinal prostheses. Sensors (Basel) 2015, 15, 14345–14355. [Google Scholar] [CrossRef]
- Lee, Y.T.; Lin, C.W.; Lin, C.M.; Yeh, S.R.; Chang, Y.C.; Fu, C.C.; Fang, W. A 3D glass microprobe array with embedded silicon for alignment and electrical connection. In Proceedings of the Solid-state Sensors, Actuators & Microsystems Conference, Denver, CO, USA, 21–25 June 2009. [Google Scholar]
- Jones, K.E.; Campbell, P.K.; Normann, R.A. A glass/silicon composite intracortical electrode array. Ann. Biomed. Eng. 1992, 20, 423–437. [Google Scholar] [CrossRef]
- Topper, M.; Klein, M.; Buschick, K.; Glaw, V.; Solzbacher, F. Biocompatible hybrid flip chip microsystem integration for next generation wireless neural interfaces. In Proceedings of the Electronic Components & Technology Conference, San Diego, CA, USA, 30 May–2 June 2006. [Google Scholar]
- Lin, C.-W.; Hsu, C.-P.; Yang, H.-A.; Wang, W.C.; Fang, W. Implementation of silicon-on-glass MEMS devices with embedded through-wafer silicon vias using the glass reflow process for wafer-level packaging and 3D chip integration. J. Micromech. Microeng. 2008, 18, 025018. [Google Scholar] [CrossRef]
- Sohee, K.; Tathireddy, P.; Normann, R.A.; Solzbacher, F. Thermal impact of an active 3-D microelectrode array implanted in the brain. IEEE Trans. Neural Syst. Rehab. Eng. 2007, 15, 493–501. [Google Scholar] [CrossRef]
- Kim, S.; Zoschke, K.; Klein, M.; Black, D.; Buschick, K.; Toepper, M.; Tathireddy, P.; Harrison, R.; Oppermann, H.; Solzbacher, F. Switchable polymer-based thin film coils as a power module for wireless neural interfaces. Sensor. Actuators A (Phys.) 2007, 136, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Matin, M.A.; Ikedo, A.; Kawano, T.; Sawada, K.; Ishida, M. Microscale temperature sensing using novel reliable silicon vertical microprobe array: Computation and experiment. Microelectron. Reliab. 2015, 55, 2689–2697. [Google Scholar] [CrossRef]
- Campbell, P.K.; Jones, K.E.; Huber, R.J.; Horch, K.W.; Normann, R.A. A silicon-based, three-dimensional neural interface: Manufacturing processes for an intracortical electrode array. IEEE Trans. Biomed. Eng. 1991, 38, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.J.; Steinmetz, N.A.; Siegle, J.H.; Denman, D.J.; Bauza, M.; Barbarits, B.; Lee, A.K.; Anastassiou, C.A.; Andrei, A.; Aydın, Ç.J.N. Fully integrated silicon probes for high-density recording of neural activity. Nature 2017, 551, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Jonathan, V.; Dae-Hyeong, K.; Moss, J.D.; Yun-Soung, K.; Blanco, J.A.; Nicholas, A.; Andrew, H.; Xiao, J.; Huang, Y.; Callans, D.J. A conformal, bio-interfaced class of silicon electronics for mapping cardiac electrophysiology. Sci. Transl. Med. 2010, 2, 22–24. [Google Scholar]
- Usmanov, D.T.; Ninomiya, S.; Hiraoka, K.; Wada, H.; Nakano, H.; Matsumura, M.; Sanada-Morimura, S.; Nonami, H. Electrospray generated from the tip-sealed fine glass capillary inserted with an acupuncture needle electrode. J. Am. Soc. Mass. Spectrom. 2018, 29, 2297–2304. [Google Scholar] [CrossRef] [PubMed]
- Stavrinidis, G.; Michelakis, K.; Kontomitrou, V.; Giannakakis, G.; Sevrisarianos, M.; Sevrisarianos, G.; Chaniotakis, N.; Alifragis, Y.; Konstantinidis, G. SU-8 microneedles based dry electrodes for electroencephalogram. Microelectron. Eng. 2016, 159, 114–120. [Google Scholar] [CrossRef]
- Herwik, S.; Holzhammer, T.; Paul, O.; Ruther, P. Out-of-plane assembly of 3D neural probe arrays using a platform with SU8-based thermal actuators. In Proceedings of the Solid-state Sensors, Actuators & Microsystems Conference, Beijing, China, 5–9 June 2011. [Google Scholar]
- Martinez, V.; Behr, P.; Drechsler, U.; Polesel-Maris, J.; Potthoff, E.; Vörös, J.; Zambelli, T. SU-8 hollow cantilevers for AFM cell adhesion studies. J. Micromech. Microeng. 2016, 26, 055006. [Google Scholar] [CrossRef] [Green Version]
- Takano, N.; Tachikawa, H.; Miyano, T.; Nishiyabu, K. Insertion testing of polyethylene glycol microneedle array into cultured human skin with biaxial tension. J. Sol. Mech. Mater. Eng. 2009, 3, 604–612. [Google Scholar] [CrossRef]
- Tan, K.L.; Cheng, M.Y.; Chen, W.G.; Rui, Q.L.; Damalerio, M.R.B.; Yao, L.; Li, P.; Gu, Y.D.; Min, K.J. Polyethylene glycol-coated polyimide-based probe with neural recording IC for chronic neural recording. Adv. Mater. Res. 2013, 849, 183–188. [Google Scholar] [CrossRef]
- Chen, P.C.; Lal, A. Detachable ultrasonic enabled inserter for neural probe insertion using biodissolvable Polyethylene Glycol. In Proceedings of the Transducers-International Conference on Solid-state Sensors, Anchorage, AK, USA, 21–25 June 2015. [Google Scholar]
- Zhou, K.; Li, Y.; Peng, Y.; Cui, X.; Dai, J.; Cui, M. Structure-property relationships of polyethylene glycol modified fluorophore as near-Infrared abeta imaging probes. Anal. Chem. 2018, 90, 8576–8582. [Google Scholar] [CrossRef]
- Follstaedt, D.M.; Yost, F.G.; Pope, L.E.; Picraux, S.T.; Knapp, J.A. The amorphous phase and surface mechanical properties of 304 stainless steel implanted with Ti and C. Appl. Phys. Lett. 1983, 43, 358–360. [Google Scholar] [CrossRef]
- Xia, Y.T.; Liu, X.; Han, D.; Han, Z.F. Improvement of mechanical properties of martensitic stainless steel by plasma nitriding at low temperature. Acta. Metall. Sin. (Engl. Lett.) 2008, 21, 21–29. [Google Scholar] [CrossRef]
- Nie, F.L.; Wang, S.G.; Wang, Y.B.; Wei, S.C.; Zheng, Y.F. Comparative study on corrosion resistance and in vitro biocompatibility of bulk nanocrystalline and microcrystalline biomedical 304 stainless steel. Dent. Mater. 2011, 27, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Ge, S.; Yang, S.; Wang, H.; Dong, Q.; Qin, Z.; Gao, J.; Yong, W. Study on the biodegradability and biocompatibility of WE magnesium alloys. Mater. Sci. Eng. C 2012, 32, 2190–2198. [Google Scholar] [CrossRef]
- In, S.L.; Gwak, Y.S.; Kim, H.R.; Razzaq, A.; Lee, K.S.; Kim, H.Y.; Chang, S.; Lee, B.H.; Grimes, C.A.; Yang, C.H. Hierarchical micro/nano-porous acupuncture needles offering enhanced therapeutic properties. Sci. Rep. 2016, 6, 34061. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Farris, R.J. The characterization of thermal and elastic constants for an epoxy photoresist SU8 coating. J. Mater. Sci. 2002, 37, 4793–4799. [Google Scholar] [CrossRef]
- Athanasoulia, I.-G.; Tarantili, P.A. Preparation and characterization of polyethylene glycol/poly(L-lactic acid) blends. Pure Appl. Chem. 2017, 89, 141–152. [Google Scholar] [CrossRef]
- Internet SU-8 2000 Permanent Epoxy Negative Photoresist processing guidelines for:SU-8 2000.5, SU-8 2002, SU-8 2005, SU-8 2007, SU-8 2010 and SU-8 2015 [internet]. Available online: http://www.microchem.com/Prod-SU82000.htm (accessed on 8 January 2019).
- Fan, Y.; Yang, N.; Huo, X.; Xu, S. Thermal sensing in fluid at the micro-nano-scales. Biomicrofluidics 2018, 12, 041501. [Google Scholar]
- Yang, F.; Li, G.; Yang, J.; Wang, Z.; Han, D.; Zheng, F.; Xu, S. Measurement of local temperature increments induced by cultured HepG2 cells with micro-thermocouples in a thermally stabilized system. Sci. Rep. 2017, 7, 1721. [Google Scholar] [CrossRef]
- Han, D.; Li, G.; Zhou, S.; Wang, Z.; Yang, F.; Xu, S. To save half contact pads in 2D mapping of local temperatures with a thermocouple array. RSC Adv. 2017, 7, 9100–9105. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Sun, W.; Chen, Q.; Xu, S. Thin-film thermocouple array for time-resolved local temperature mapping. IEEE Electr. Device Lett. 2011, 32, 1606–1608. [Google Scholar] [CrossRef]
- Xu, J.J.; Lei, Z.J.; Guo, J.K.; Huang, J.; Wang, W.; Reibetanz, U.; Xu, S.Y. Trapping and driving individual charged micro-particles in fluid with an electrostatic device. Nanomicro Lett. 2016, 8, 270–281. [Google Scholar] [CrossRef]
- Han, D.H.; Xu, J.J.; Wang, Z.H.; Yang, N.; Li, X.Z.; Qian, Y.Y.; Li, G.; Dai, R.J.; Xu, S. Penetrating effect of high-intensity infrared laser pulses through body tissue. RSC Adv. 2018, 8, 32344–32357. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, F.; Han, D.; Li, G.; Xu, J.; Xu, S. Alternative method to fabricate microdevices on a freestanding Si3N4 window. J. Vac. Sci. Technol. B 2017, 35, 041601. [Google Scholar] [CrossRef]
- Li, G.; Wang, Z.; Mao, X.; Zhang, Y.; Huo, X.; Liu, H.; Xu, S. Real-time two-dimensional mapping of relative local surface temperatures with a thin-film sensor array. Sensors (Basel) 2016, 16, 977. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, N.; Wang, Z.; Xu, J.; Gui, L.; Tang, Z.; Zhang, Y.; Yi, M.; Yue, S.; Xu, S. Multifunctional Freestanding Microprobes for Potential Biological Applications. Sensors 2019, 19, 2328. https://doi.org/10.3390/s19102328
Yang N, Wang Z, Xu J, Gui L, Tang Z, Zhang Y, Yi M, Yue S, Xu S. Multifunctional Freestanding Microprobes for Potential Biological Applications. Sensors. 2019; 19(10):2328. https://doi.org/10.3390/s19102328
Chicago/Turabian StyleYang, Nana, Zhenhai Wang, Jingjing Xu, Lijiang Gui, Zhiqiang Tang, Yuqi Zhang, Ming Yi, Shuanglin Yue, and Shengyong Xu. 2019. "Multifunctional Freestanding Microprobes for Potential Biological Applications" Sensors 19, no. 10: 2328. https://doi.org/10.3390/s19102328




