Multiplex Detection of Different Magnetic Beads Using Frequency Scanning in Magnetic Frequency Mixing Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Magnetic Beads
2.2. Principles of Magnetic Frequency Mixing Detection
2.3. Magnetic Frequency Mixing Detection Setup
2.4. Sample Preparation
2.4.1. Preparation of the Immunofiltration Columns
2.4.2. Fixation of the Beads to the Surface of the Immunofiltration Columns
2.5. Measurement Procedure
2.5.1. Performing the Measurements
2.5.2. Data Processing and Handling
3. Results and Discussion
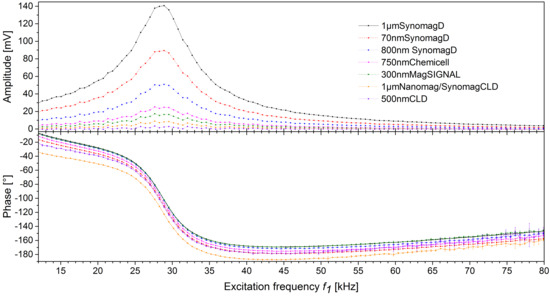
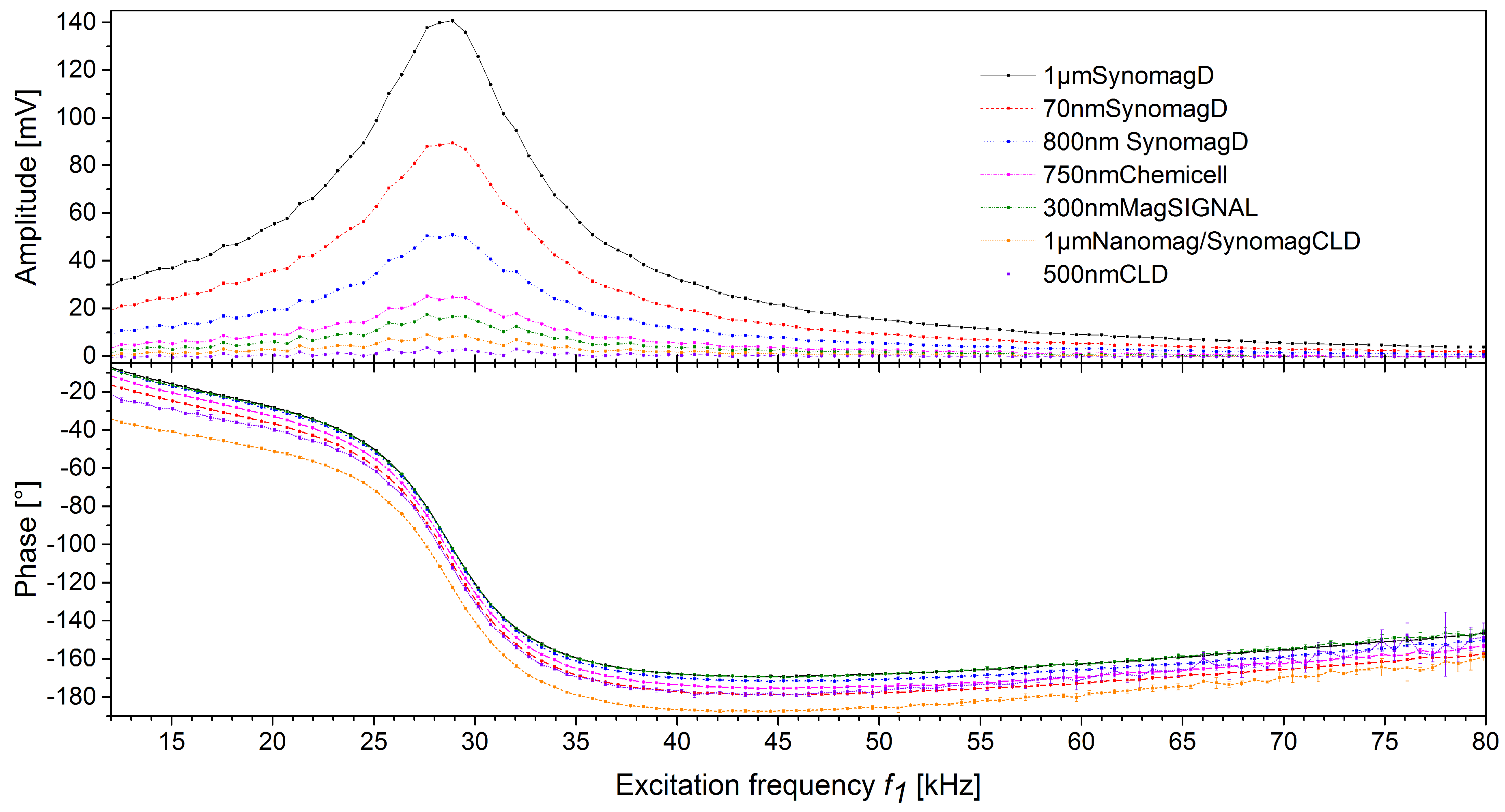
3.1. Excitation Frequency Scan of Different MBs
3.2. Effect of Amount of MBs Fixed to the Filter
3.3. Measurement of Samples Containing Two Different MBs
3.4. Measurement of Mixtures with Amplitude Reduced MB Solution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
- : as a, b are element of ℕ also there sum is an element of ℕ: (b + a) ∈ ℕ.
- : as a, b are element of ℕ also there sum is an element of ℕ if b > a: (b − a) ∈ ℕ, if b > a.
References
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Kolhatkar, A.G.; Zenasni, O.; Xu, S.; Lee, T.R. Biosensing using magnetic particle detection techniques. Sensors 2017, 17, 2300. [Google Scholar] [CrossRef] [PubMed]
- Walper, S.A.; Lasarte Aragonés, G.; Sapsford, K.E.; Brown, C.W.; Rowland, C.E.; Breger, J.C.; Medintz, I.L. Detecting biothreat agents: From current diagnostics to developing sensor technologies. ACS Sens. 2018, 3, 1894–2024. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.S.; Mayo, M.W.; Bruno, J.G.; Bronk, B.V.; Batt, C.A.; Chambers, J.P. A review of molecular recognition technologies for detection of biological threat agents. Biosens. Bioelectron. 2000, 15, 549–578. [Google Scholar] [CrossRef]
- Dorst, B.V.; Mehta, J.; Bekaert, K.; Rouah-Martin, E.; Coen, W.D.; Dubruel, P.; Blust, R.; Robbens, J. Recent advances in recognition elements of food and environmental biosensors: A review. Biosens. Bioelectron. 2010, 26, 1178–1194. [Google Scholar] [CrossRef] [PubMed]
- Besse, P.-A.; Boero, G.; Demierre, M.; Pott, V.; Popovic, R. Detection of a single magnetic microbead using a miniaturized silicon hall sensor. Appl. Phys. Lett. 2002, 80, 4199–4201. [Google Scholar] [CrossRef]
- Choi, J.; Gani, A.W.; Bechstein, D.J.B.; Lee, J.-R.; Utz, P.J.; Wang, S.X. Portable, one-step and rapid GMR biosensor platform with smartphone interface. Biosens. Bioelectron. 2016, 85, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chieh, J.J.; Yang, S.-Y.; Horng, H.-E.; Yu, C.Y.; Lee, C.L.; Wu, H.L.; Hong, C.-Y.; Yang, H.-C. Immunomagnetic reduction assay using high-tc superconducting-quantum-interference-device-based magnetosusceptometry. J. Appl. Phys. 2010, 107, 074903. [Google Scholar] [CrossRef]
- Kötitz, R.; Matz, H.; Trahms, L.; Koch, H.; Weitschies, W.; Rheinländer, T.; Semmler, W.; Bunte, T. Squid based remanence measurements for immunoassays. IEEE Trans. Appl. Supercond. 1997, 7, 3678–3681. [Google Scholar] [CrossRef]
- Astalan, A.P.; Ahrentorp, F.; Johansson, C.; Larsson, K.; Krozer, A. Biomolecular reactions studied using changes in brownian rotation dynamics of magnetic particles. Biosens. Bioelectron. 2004, 19, 945–951. [Google Scholar] [CrossRef]
- Lee, H.; Sun, E.; Ham, D.; Weissleder, R. Chip–nmr biosensor for detection and molecular analysis of cells. Nat. Med. 2008, 14, 869–874. [Google Scholar] [CrossRef]
- Krause, H.-J.; Wolters, N.; Zhang, Y.; Offenhäusser, A.; Miethe, P.; Meyer, M.H.F.; Hartmann, M.; Keusgen, M. Magnetic particle detection by frequency mixing for immunoassay applications. J. Magn. Magn. Mater. 2007, 311, 436–444. [Google Scholar] [CrossRef]
- Hong, C.-Y.; Wu, C.C.; Chiu, Y.C.; Yang, S.Y.; Horng, H.E.; Yang, H.C. Magnetic susceptibility reduction method for magnetically labeled immunoassay. Appl. Phys. Lett. 2006, 88, 212512. [Google Scholar] [CrossRef]
- Meyer, M.H.F.; Krause, H.-J.; Hartmann, M.; Miethe, P.; Oster, J.; Keusgen, M. Francisella tularensis detection using magnetic labels and a magnetic biosensor based on frequency mixing. J. Magn. Magn. Mater. 2007, 311, 259–263. [Google Scholar] [CrossRef]
- Meyer, M.H.F.; Hartmann, M.; Krause, H.-J.; Blankenstein, G.; Mueller-Chorus, B.; Oster, J.; Miethe, P.; Keusgen, M. CRP determination based on a novel magnetic biosensor. Biosens. Bioelectron. 2007, 22, 973–979. [Google Scholar] [CrossRef]
- Rettcher, S.; Jungk, F.; Kühn, C.; Krause, H.-J.; Nölke, G.; Commandeur, U.; Fischer, R.; Schillberg, S.; Schröper, F. Simple and portable magnetic immunoassay for rapid detection and sensitive quantification of plant viruses. Appl. Environ. Microbiol. 2015, 81, 3039–3048. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; Luong, J.H.T. Chapter 1—Immunoassays: An overview. In Handbook of Immunoassay Technologies; Vashist, S.K., Luong, J.H.T., Eds.; Academic Press: New York, NY, USA, 2018; pp. 1–18. [Google Scholar]
- Vashist, S.K.; Luong, J.H.T. Chapter 17—Immunoassays: Future prospects and possibilities. In Handbook of Immunoassay Technologies; Vashist, S.K., Luong, J.H.T., Eds.; Academic Press: New York, NY, USA, 2018; pp. 455–466. [Google Scholar]
- Lenglet, L. Multiparametric magnetic immunoassays utilizing non-linear signatures of magnetic labels. J. Magn. Magn. Mater. 2009, 321, 1639–1643. [Google Scholar] [CrossRef]
- Achtsnicht, S.; Tödter, J.; Niehues, J.; Telöken, M.; Offenhäusser, A.; Krause, H.-J.; Schröper, F. 3D printed modular immunofiltration columns for frequency mixing-based multiplex magnetic immunodetection. Sensors 2019, 19, 148. [Google Scholar] [CrossRef]
- Shasha, C.; Teeman, E.; Krishnan, K.M. Harmonic simulation study of simultaneous nanoparticle size and viscosity differentiation. IEEE Magn. Lett. 2017, 8, 1–5. [Google Scholar] [CrossRef]
- Coene, A.; Leliaert, J.; Liebl, M.; Löwa, N.; Steinhoff, U.; Crevecoeur, G.; Dupré, L.; Wiekhorst, F. Multi-color magnetic nanoparticle imaging using magnetorelaxometry. Phys. Med. Biol. 2017, 62, 3139–3157. [Google Scholar] [CrossRef]
- Wu, K.; Batra, A.; Jain, S.; Ye, C.; Liu, J.; Wang, J.-P. A simulation study on superparamagnetic nanoparticle based multi-tracer tracking. Appl. Phys. Lett. 2015, 107, 173701. [Google Scholar] [CrossRef]
- Wu, K.; Wang, Y.; Feng, Y.; Yu, L.; Wang, J.-P. Colorize magnetic nanoparticles using a search coil based testing method. J. Magn. Magn. Mater. 2015, 380, 251–254. [Google Scholar] [CrossRef]
- Lak, A.; Thünemann, A.F.; Schilling, M.; Ludwig, F. Resolving particle size modality in bi-modal iron oxide nanoparticle suspensions. J. Magn. Magn. Mater. 2015, 380, 140–143. [Google Scholar] [CrossRef]
- Häfeli, U.O.; Ciocan, J.P.D.R. Characterization of magnetic particles and microspheres and their magnetophoretic mobility using a digital microscopy method. Eur. Cells Mater. 2002, 3 (Suppl. 2), 24–27. [Google Scholar]
- Zhou, C.; Boland, E.D.; Todd, P.W.; Hanley, T.R. Magnetic particle characterization—Magnetophoretic mobility and particle size. Cytom. Part A 2016, 89, 585–593. [Google Scholar] [CrossRef]
- Achtsnicht, S.; Schönenborn, K.; Offenhäusser, A.; Krause, H.-J. Measurement of the magnetophoretic velocity of different superparamagnetic beads. J. Magn. Magn. Mater. 2019, 477, 244–248. [Google Scholar] [CrossRef]
- Bender, P.; Balceris, C.; Ludwig, F.; Posth, O.; Bogart, L.K.; Szczerba, W.; Castro, A.; Nilsson, L.; Costo, R.; Gavilán, H.; et al. Distribution functions of magnetic nanoparticles determined by a numerical inversion method. New J. Phys. 2017, 19, 073012. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, F.; Balceris, C.; Viereck, T.; Posth, O.; Steinhoff, U.; Gavilan, H.; Costo, R.; Zeng, L.; Olsson, E.; Jonasson, C.; et al. Size analysis of single-core magnetic nanoparticles. J. Magn. Magn. Mater. 2017, 427, 19–24. [Google Scholar] [CrossRef]





| Bead Solution | Amplitude | Phase | |||
|---|---|---|---|---|---|
| Name | Volume [µL] | Mean [mV] | Standard Deviation [mV] | Mean [°] | Standard Deviation [°] |
| C1 | 6 | 46.88 | 0.008 | 307.7 | 0.231 |
| C2 | 8 | 54.39 | 0.009 | 308.8 | 0.245 |
| C3 | 10 | 68.07 | 0.014 | 308.8 | 0.211 |
| Mixture | |||||||
|---|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M4 | M5 | |||
| Bead Type | A | 1µmNanomag/Synomag CLD | 10 µL | 7.5 µL | 5 µL | 2.5 µL | 0 µL |
| B | 1µmSynomagD | 0 µL | 2.5 µL | 5 µL | 7.5 µL | 10 µL | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Achtsnicht, S.; Pourshahidi, A.M.; Offenhäusser, A.; Krause, H.-J. Multiplex Detection of Different Magnetic Beads Using Frequency Scanning in Magnetic Frequency Mixing Technique. Sensors 2019, 19, 2599. https://doi.org/10.3390/s19112599
Achtsnicht S, Pourshahidi AM, Offenhäusser A, Krause H-J. Multiplex Detection of Different Magnetic Beads Using Frequency Scanning in Magnetic Frequency Mixing Technique. Sensors. 2019; 19(11):2599. https://doi.org/10.3390/s19112599
Chicago/Turabian StyleAchtsnicht, Stefan, Ali Mohammad Pourshahidi, Andreas Offenhäusser, and Hans-Joachim Krause. 2019. "Multiplex Detection of Different Magnetic Beads Using Frequency Scanning in Magnetic Frequency Mixing Technique" Sensors 19, no. 11: 2599. https://doi.org/10.3390/s19112599
APA StyleAchtsnicht, S., Pourshahidi, A. M., Offenhäusser, A., & Krause, H.-J. (2019). Multiplex Detection of Different Magnetic Beads Using Frequency Scanning in Magnetic Frequency Mixing Technique. Sensors, 19(11), 2599. https://doi.org/10.3390/s19112599