Malaria-Infected Red Blood Cell Analysis through Optical and Biochemical Parameters Using the Transport of Intensity Equation and the Microscope’s Optical Properties
Abstract
:1. Introduction
2. Materials and Methods
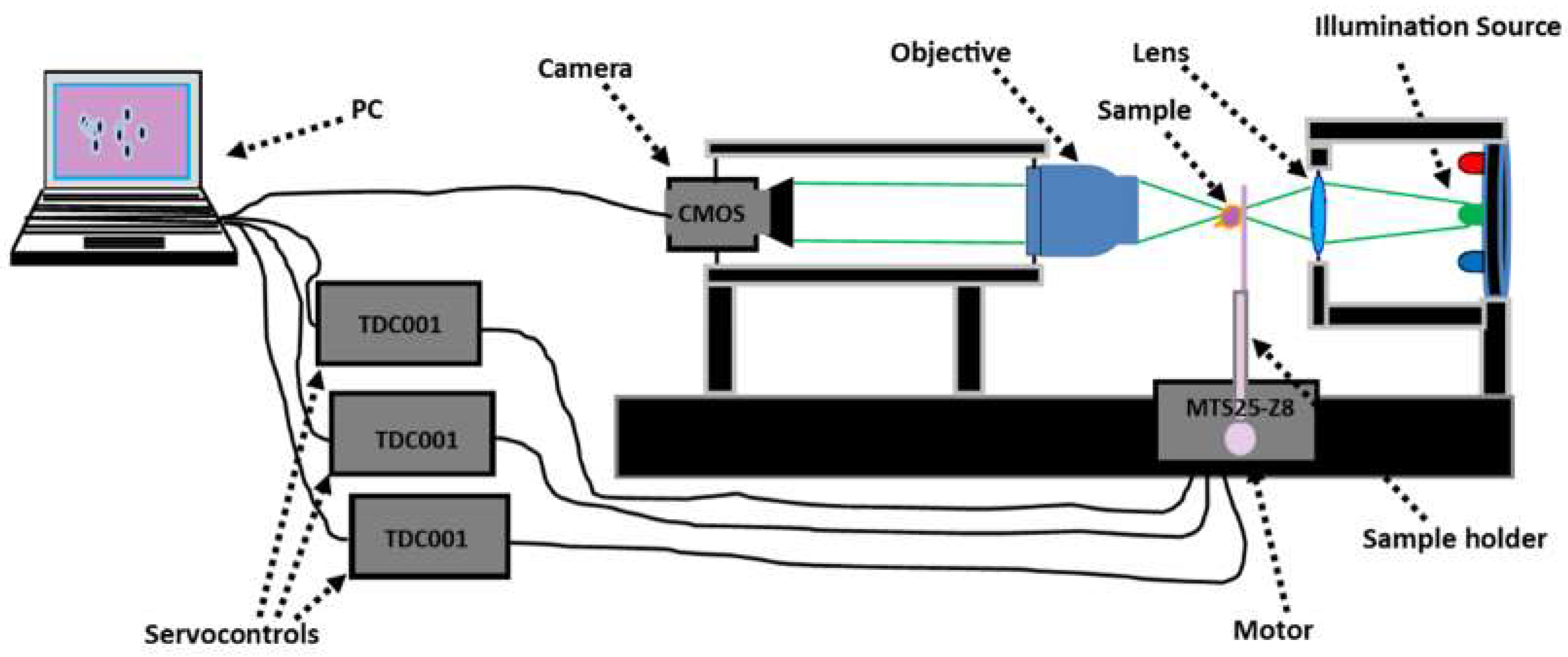
2.1. Experimental Set-Up
2.2. Sample Preparation
2.3. Measurement Procedure
- : the image of the sample (sample measurement);
- : the image of an empty slide (reference measurement);
- : the image of the background which was taken with no slide or sample and with the source illumination turned off.
2.4. Analysis Methods
- The phase of the wave field traversing the pure phase sample at the wavelength wthin a plane normal to the optical axis was computed using Equation (4) [29,30,31]:and are the Fourier Transform and the inverse Fourier Transform respectively. The parameters are the spatial frequency variables corresponding to the coordinates while is the intensity of the wave field within a z plane normal to the optical axis.
- The refractive index of each point within the sample is defined by Equation (5) [18]:where is the refractive index of the specimen layer. To retrieve the topography of the sample, we used Equation (6) [32]:is the phase which corresponds to the focal plane and is the mean value of the refractive index within the sample in the focal plane.
- We performed measurements at the wavelength λ = 800 nm, because at this wavelength the RBC is a pure phase object [33], making it possible to perform linear deconvolution according to Equation (2). The RBCs essentially consist of hemoglobin (32%), the surrounding membrane (3%) and water (65%) [34]. As such, they can be considered as aqueous solutions whose main solute is the hemoglobin. Therefore, the refractive index of the cell is essentially due to the hemoglobin concentration within the RBC [18,35]. Friebel and Meinke [36] and Tycko et al. [35] showed that a variation in hemoglobin concentration leads to a variation in the refractive index of the cell. These two parameters can be used to confirm whether or not RBCs are healthy or parasitized. The hemoglobin concentration within the RBC is deduced from its refractive index distribution using [36]:where is the RBC’s hemoglobin concentration, at wavelength λ = 800 nm and is the refractive index of water.
3. Results and Discussion
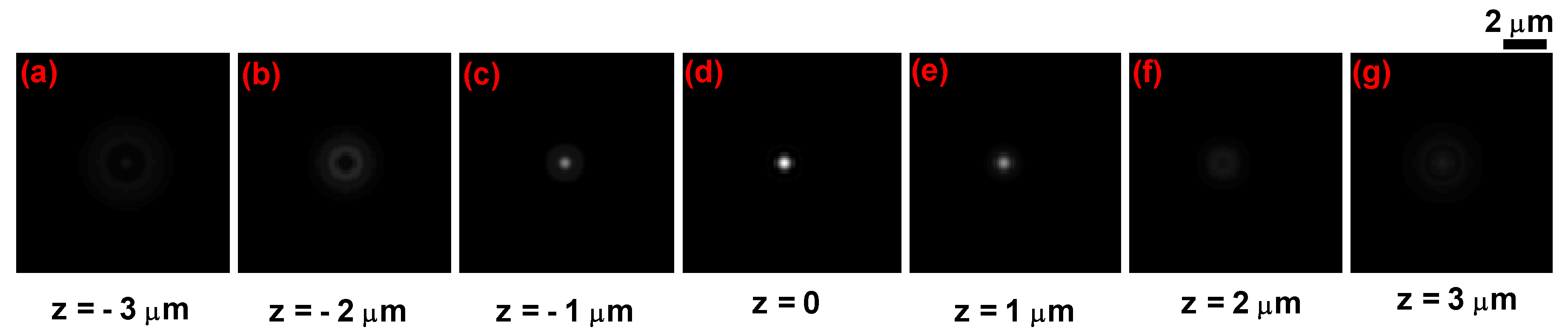
3.1. Application to Healthy Red Blood Cells
3.2. Application to Malaria-Infected Red Blood Cells
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Noppadon, T.; Chatnapa, D.; Polrat, W.; Srivicha, K. Malaria Diagnosis, A Brief Review. Korean J. Parasitol. 2009, 47, 93–102. [Google Scholar] [CrossRef]
- Gentilini, M. Maladies parasitaires. In Médecine Tropicale, 5th ed.; 2ème Tirage Actualisé; Flammarion Médecine Science: Paris, France, 1995; pp. 51–122. [Google Scholar]
- Ogouyèmi, H.A.; Kinde, G.; Keke, C.; Gonçalves, E.; Alapini, N.; Adjovi, F.; Adisso, L.; Bossou, C.; Denon, Y.V.; Massougbodji, A. Évaluation d’un test de diagnostic rapide et d’un microscope à fluorescence portable pour le diagnostic du paludisme à Cotonou (Bénin). Bull. Soc. Pathol. Exot. 2013, 106, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, S.; Lee, M.; Oh, J.; Yang, S.A.; Park, Y. Refractive index as an intrinsic imaging contrast for 3D label-free live cell imaging. bioRxiv 2017. [Google Scholar] [CrossRef]
- Craig, M.H.; Sharp, B.L. Comparative evaluation of four techniques for the diagnosis of Plasmodium falciparum infections. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 279–282. [Google Scholar] [CrossRef]
- Mvumbi, D.M.; Boreux, R.; Mvumbi, G.L.; Bobanga, T.L.; Situakibanza, H.; Melin, P.; Kayembe, J.M.N.; DeMol, P.; Hayette, M.P. Diagnostic du paludisme sévère, Comparaison de la technique de PCR en temps réel versus microscopie à Kinshasa, République démocratique du Congo. Ann. Afr. Med. 2015, 8, 2044–2051. [Google Scholar]
- Park, Y.; Diez-Silva, M.; Popescu, G.; Lykotrafitis, G.; Choi, W.; Feld, M.S.; Suresh, S. Refractive index maps and membrane dynamics of human red blood cells parasitized by Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 2008, 105, 13730–13735. [Google Scholar] [CrossRef] [PubMed]
- Kyoohyun, K.; HyeOk, Y.; Diez-Silva, M.; Ming, D.; Ramachandra, R.D.; YongKeun, P. High-resolution three-dimensional imaging of red blood cells parasitized by Plasmodium falciparum and in situ hemozoin crystals using optical diffraction tomography. J. Biomed. Opt. 2014, 19, 011005. [Google Scholar]
- Jang, Y.; Jang, J.; YongKeun, P. Dynamic spectroscopic phase microscopy for quantifying hemoglobin concentration and dynamic membrane fluctuation in red blood cells. Opt. Express 2012, 9, 9673–9681. [Google Scholar] [CrossRef]
- Zhuo, W.; Krishnara, T.; Balla, A.; Popescu, G. Tissue refractive index as marker of disease. J. Biomed. Opt. 2011, 16, 116017. [Google Scholar]
- Charrière, F.; Marian, A.; Montfort, F.; Kuehn, J.; Colomb, T.; Cuche, E.; Marquet, P.; Depeursinge, C. Cell refractive index tomography by digital holographic microscopy. Opt. Lett. 2006, 31, 178–180. [Google Scholar] [CrossRef]
- Lee, K.; Kim, K.; Jung, J.; Heo, J.; Cho, S.; Lee, S.; Chang, G.; Jo, Y.; Park, H.; Park, Y. Quantitative phase imaging techniques for the study of cell pathophysiology, from principles to applications. Sensors 2013, 4, 4170–4191. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, P.; Takahiro, I.; Ramachandra, R.D.; Feld, M.S. Diffraction phase microscopy for quantifying cell structure and dynamics. Opt. Lett. 2006, 6, 775–777. [Google Scholar]
- Takahiro, I.; Gabriel, P.; Ramachandra, R.D.; Feld, M.S. Hilbert phase microscopy for investigating fast dynamics in transparent systems. Opt. Lett. 2005, 10, 1165–1168. [Google Scholar]
- YongKeun, P.; Wonshik, C.; Zahid, Y.; Ramachandra, D.; Kamran, B.; Feld, M.S. Speckle-field digital holographic microscopy. Opt. Express 2009, 15, 12285–12292. [Google Scholar]
- YongKeun, P.; Toyohiko, Y.; Wonshik, C.; Ramachandra, D.; Feld, M.S. Spectroscopic phase microscopy for quantifying hemoglobin concentrations in intact red blood cells. Opt. Lett. 2009, 23, 3668–3670. [Google Scholar]
- Chao, Z.; Qian, C.; Anand, A. Transport of intensity equation, a new approach to phase and light field. Proc. SPIE 2014, 9271, 92710H. [Google Scholar]
- Kevin, G.P.; Steven, L.J.; Owen, J.T. Measurement of Single Cell Refractive Index; Dry Mass; Volume; and Density Using a Transillumination Microscope. Phys. Rev. Lett. 2012, 109, 118105. [Google Scholar]
- Agnero, M.A.; Zoueu, J.T.; Konan, K. Characterization of a Multimodal and Multispectral Led Imager, Application to Organic Polymer’s Microspheres with Diameter Φ = 10.2 μm. Opt. Photonics J. 2016, 6, 171–183. [Google Scholar] [CrossRef]
- Brydegaard, M.; Merdasa, A.; Jayaweera, H.; Ålebring, J.; Svanberg, S. Versatile multispectral microscope based on light emitting diodes. Rev. Sci. Instrum. 2011, 12, 123106. [Google Scholar] [CrossRef]
- Hernandez, C.C.N.; Gutierrez-Medina, B. Direct Imaging of Phase Objects Enables Conventional Deconvolution in Bright Field Light Microscopy. PLoS ONE 2014, 2, e89106. [Google Scholar] [CrossRef]
- Agnero, M.A.; Konan, K.; Kossonou, A.; Bagui, K.O.; Zoueu, J.T. A New Method to Retrieve Three-Dimensional Refractive Index and Specimen’s Size Using Transport Intensity Equation Taking into Account Diffraction. Appl. Sci. 2018, 8, 1649. [Google Scholar] [CrossRef]
- Gibson, S.F.; Lanni, F. Experimental test of an analytical model of aberration in an oil-immersion objective lens used in three-dimensional light microscopy. J. Opt. Soc. Am. A 1992, 9, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Aguet, F.; Dimitri, V.D.V.; Unser, M. An accurate PSF model with few parameters for axially shift-variant deconvolution. In Proceedings of the 2008 5th IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Paris, France, 14–17 May 2008; pp. 157–160. [Google Scholar]
- James, C.O. Optical Refractive Index of Air, Dependence on Pressure; Temperature and Composition. Appl. Opt. 1967, 6, 51–59. [Google Scholar]
- Garrel, V. Réalisation D’un Instrument D’imagerie Visible à la Limite de Diffraction Pour un Grand Télescope. Ph.D. Thesis, École Doctorale d’Astronomie et d’Astrophysique, Ile de France, France, 2012; p. 11. [Google Scholar]
- Richardson, W.H. Bayesian-Based Iterative Method of Image Restoration. J. Opt. Soc. Am. 1970, 62, 55–59. [Google Scholar] [CrossRef]
- Lucy, L.B. An iterative technique for the rectification of observed distributions. Astron. J. 1974, 79, 745–749. [Google Scholar] [CrossRef]
- Gureyev, T.; Nugent, K. Rapid quantitative phase imaging using the transport of intensity equation. Opt. Commun. 1997, 133, 339–346. [Google Scholar] [CrossRef]
- Allen, L.J.; Oxley, M.P. Phase retrieval from series of images obtained by defocus variation. Opt. Commun. 2001, 199, 65–75. [Google Scholar] [CrossRef]
- Laura, W.; Lei, T.; George, B. Transport of Intensity phase-amplitude imaging with higher order intensity derivatives. Opt. Express 2010, 18, 12552–12561. [Google Scholar]
- José, A.R.; Tatiana, A. Rapid quantitative phase imaging for partially coherent light microscopy. Opt. Express 2014, 22, 13472–13483. [Google Scholar] [Green Version]
- Friebel, M.; Meinke, M.C. Determination of the complex refractive index of highly concentrated hemoglobin solutions using transmittance and reflectance measurements. J. Biomed. Opt. 2005, 10, 064019. [Google Scholar] [CrossRef]
- Mazeron, P.; Muller, S.; El, A. Deformation of erythrocytes under shear, a small angle light scattering study. Biorheology 1997, 34, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Tycko, D.H.; Metz, M.H.; Epstein, E.A.; Grinbaum, A. Flow-cymetric light scattering measurements of red blood cell volume and hemoglobin concentration. Appl. Opt. 1985, 24, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- Moritz, F.; Martina, M. Model function to calculate the refractive index of native hemoglobin in the wavelength range of 250–1100 nm dependent on concentration. Appl. Opt. 2006, 45, 2838–2842. [Google Scholar]
- Khairullina, A.Y. Regularities of regular and diffuse transmission of particle monolayers with different packing densities and optical properties. Opt. Spectrosc. 1982, 53, 623–626. [Google Scholar]
- Wang, X.-Q.; Yu, J.-T.; Wang, P.-N.; Chen, J.-Y. Light distribution in the erythrocyte under laser irradiation, a finite-difference time-domain calculation. Opt. Soc. Am. 2008, 22, 4037–4044. [Google Scholar] [CrossRef] [PubMed]
- Dabo-Niang, S.; Zoueu, J.T. Combining kriging, multispectral and multimodal microscopy to resolve malaria-infected erythrocyte contents. J. Microsc. 2012, 247, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, B. Caractérisation des Canaux Anioniques dans la Membrane du Globule Rouge Humain Infecté par Plasmodium Falciparum. Ph.D. Thesis, Université de Rennes 1, Rennes, France, 2009. [Google Scholar]
- Schmitt, T.H.; Frezzatti WA, J.; Schreier, S. Haemin-induced lipid membrane disorder and increased permeability, a molecular model for mechanism of cell lysis. Arch. Biochem. Biophys. 1993, 307, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Sugiola, Y.; Suzuki, M. The chemical basis for ferriprotoporphyrin IX-chloroquine Complex induced lipid peroxidation. Biochem. Biophys. Acta 1991, 1074, 19–24. [Google Scholar] [CrossRef]
- Sangyeon, C.; Soomin, K.; Youngchan, K.; YongKeun, P. Optical imaging techniques for the study of malaria. Trends Biotechnol. 2012, 30, 71–79. [Google Scholar]
- Yulia, M.S.; Janus, P.; Wilbur, K.M.; Luis, H.G. Quantitative analysis of morphological alterations in Plasmodium falciparum infected red blood cells through theoretical interpretation of spectral measurements. J. Theor. Biol. 2010, 265, 493–500. [Google Scholar]
- Magowan, C.; Brown, J.T.; Liang, J.; Heck, J.; Coppel, R.L.; Mohandas, N.; Meyer-Ilse, W. Intracellular structures of normal and aberrant Plasmodium falciparum malaria parasites imaged by soft X-ray microscopy. Proc. Natl. Acad. Sci. USA 1997, 94, 6222–6227. [Google Scholar] [CrossRef] [PubMed]
- Svetlana, G.; Dan, Y.; Tao, L.; Joshua, Z. Membrane Transformation du ring Malaria Parasite Release from Human Red Blood Cells. Curr. Biol. 2005, 15, 1645–1650. [Google Scholar]
- Suwanarusk, R.; Cooke, B.M.; Dondorp, A.M.; Silamut, K.; Sattabongkot, J.; White, N.J.; Udomsangpetch, R. The deformability of red blood cells parasitized by Plasmodium falciparum and P. vivax. J. Infect. Dis. 2004, 189, 190. [Google Scholar] [CrossRef] [PubMed]
- Byeon, H.; Ha, Y.R.; Lee, S.J. Holographic analysis on deformation and restoration of malaria infected red blood cells by antimalarial drug. J. Biomed. Opt. 2015, 20, 115003. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K. Theoretical models for near forward light scattering by a Plasmodium falciparum infected red blood cell. J. Mod. Opt. 2012, 59, 20–21. [Google Scholar] [CrossRef]
- Hanssen, E.; McMillan, P.J.; Leann, T. Cellular architecture of Plasmodium falciparum infected erythrocytes. Int. J. Parasitol. 2010, 40, 1127–1135. [Google Scholar] [CrossRef]








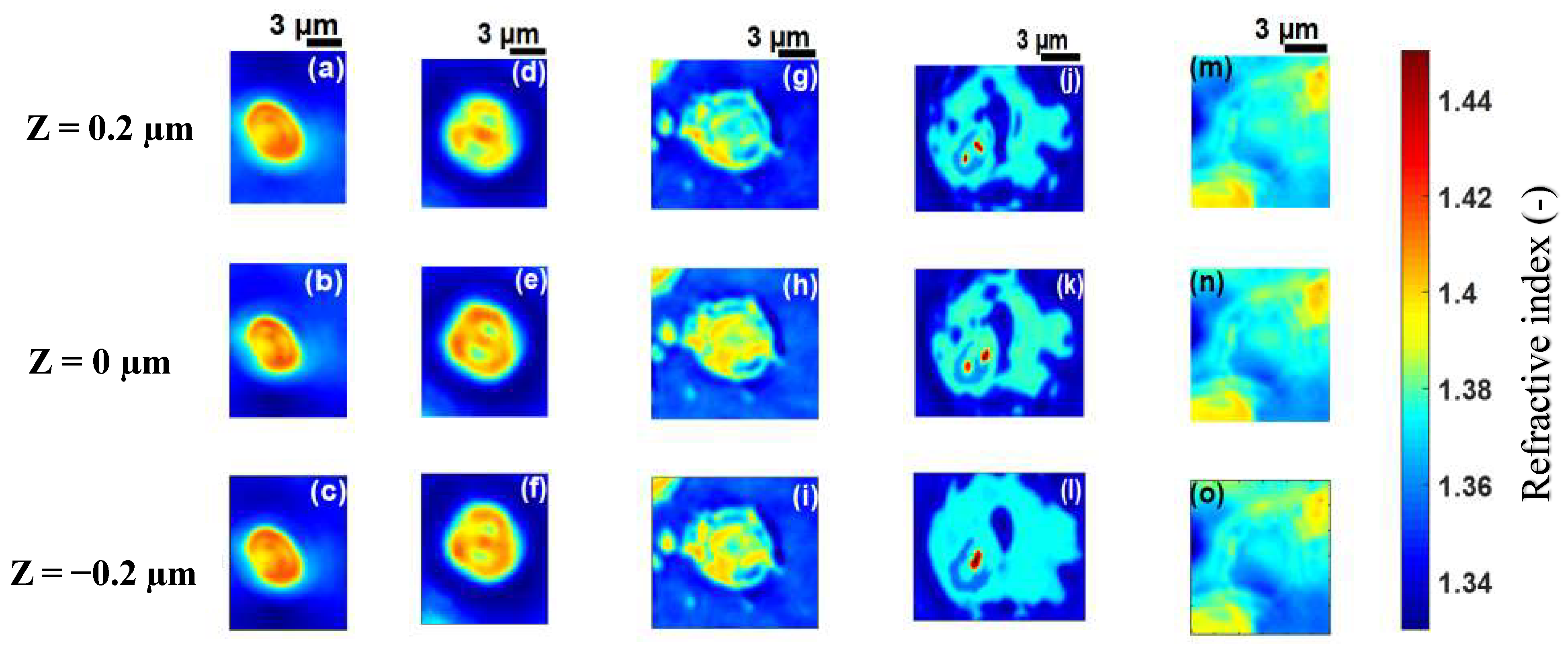
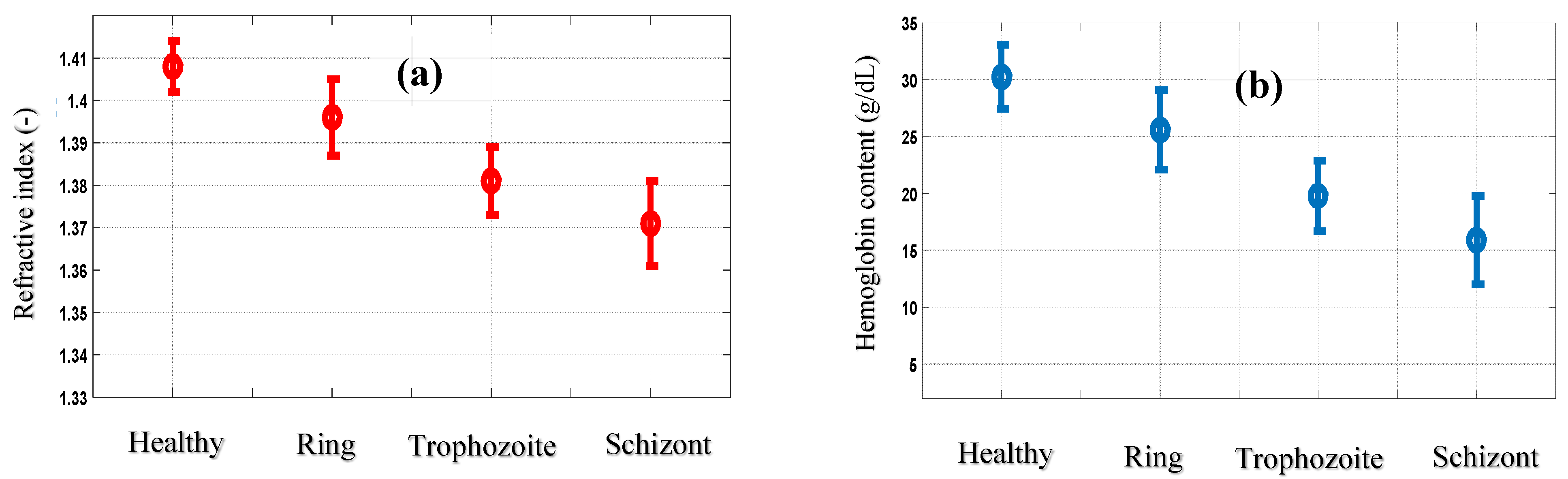
| Cell B | Cell C | Cell D | Cell E | |
|---|---|---|---|---|
| Refractive index mean values (-) | 1.396 ± 0.009 | 1.381 ± 0.008 | 1.371 ± 0.010 | 1.372 ± 0.008 |
| hemoglobin content (g/dL) | 25.59 ± 3.49 | 19.78 ± 3.10 | 15.90 ± 3.88 | 16.28 ± 3.10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agnero, M.A.; Konan, K.; Tokou, Z.G.C.S.; Kossonou, Y.T.A.; Dion, B.S.; Kaduki, K.A.; Zoueu, J.T. Malaria-Infected Red Blood Cell Analysis through Optical and Biochemical Parameters Using the Transport of Intensity Equation and the Microscope’s Optical Properties. Sensors 2019, 19, 3045. https://doi.org/10.3390/s19143045
Agnero MA, Konan K, Tokou ZGCS, Kossonou YTA, Dion BS, Kaduki KA, Zoueu JT. Malaria-Infected Red Blood Cell Analysis through Optical and Biochemical Parameters Using the Transport of Intensity Equation and the Microscope’s Optical Properties. Sensors. 2019; 19(14):3045. https://doi.org/10.3390/s19143045
Chicago/Turabian StyleAgnero, Marcel Akpa, Kouakou Konan, Zan Guy Christian Stephane Tokou, Yao Taky Alvarez Kossonou, Bienvenue Sylvère Dion, Kenneth Amiga Kaduki, and Jérémie Thouakesséh Zoueu. 2019. "Malaria-Infected Red Blood Cell Analysis through Optical and Biochemical Parameters Using the Transport of Intensity Equation and the Microscope’s Optical Properties" Sensors 19, no. 14: 3045. https://doi.org/10.3390/s19143045
APA StyleAgnero, M. A., Konan, K., Tokou, Z. G. C. S., Kossonou, Y. T. A., Dion, B. S., Kaduki, K. A., & Zoueu, J. T. (2019). Malaria-Infected Red Blood Cell Analysis through Optical and Biochemical Parameters Using the Transport of Intensity Equation and the Microscope’s Optical Properties. Sensors, 19(14), 3045. https://doi.org/10.3390/s19143045






