Transfer Printing Technology as a Straightforward Method to Fabricate Chemical Sensors Based on Tin Dioxide Nanowires
Abstract
:1. Introduction
2. Materials and Methods
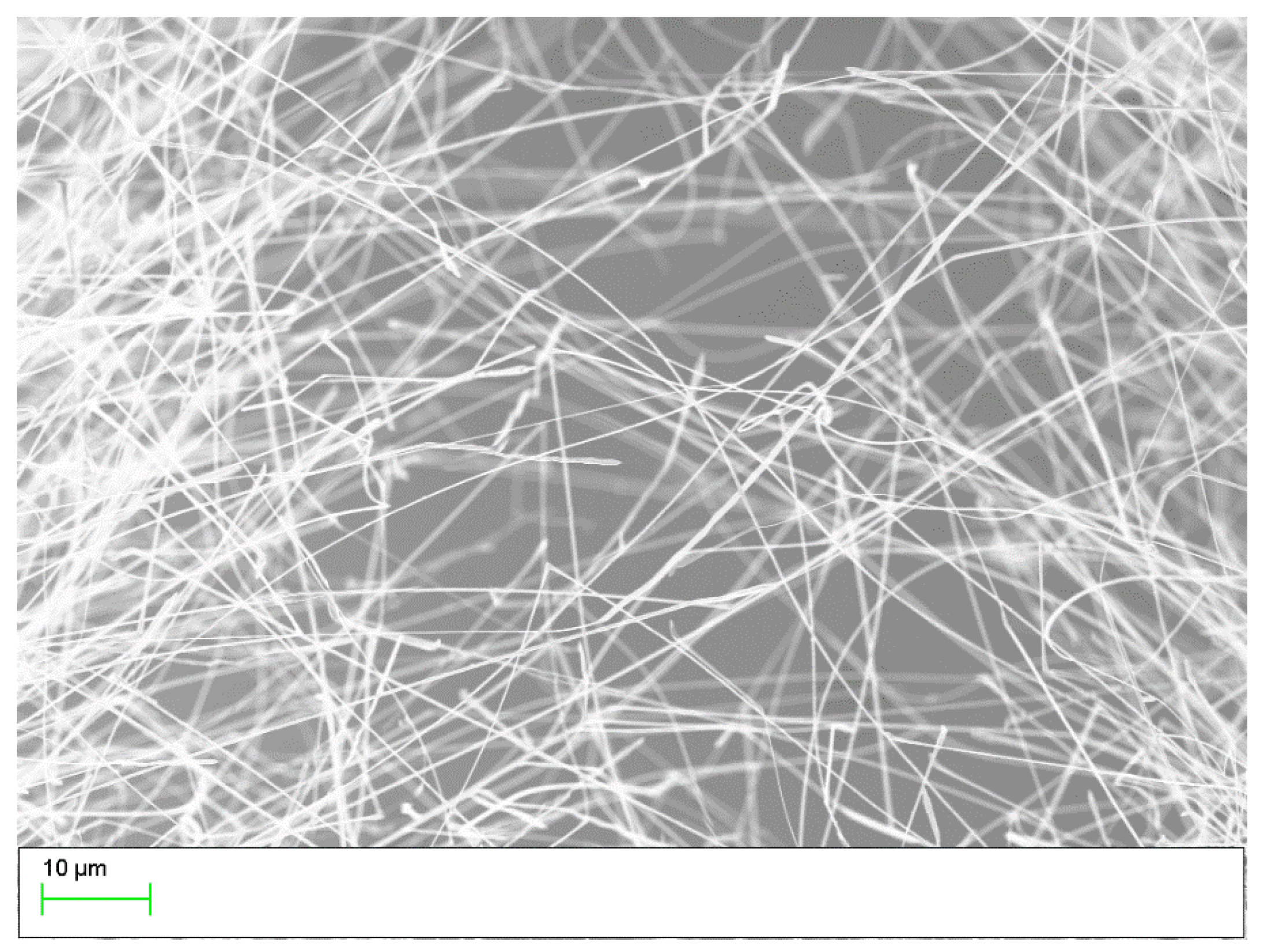
2.1. Synthesis of SnO Nanowires
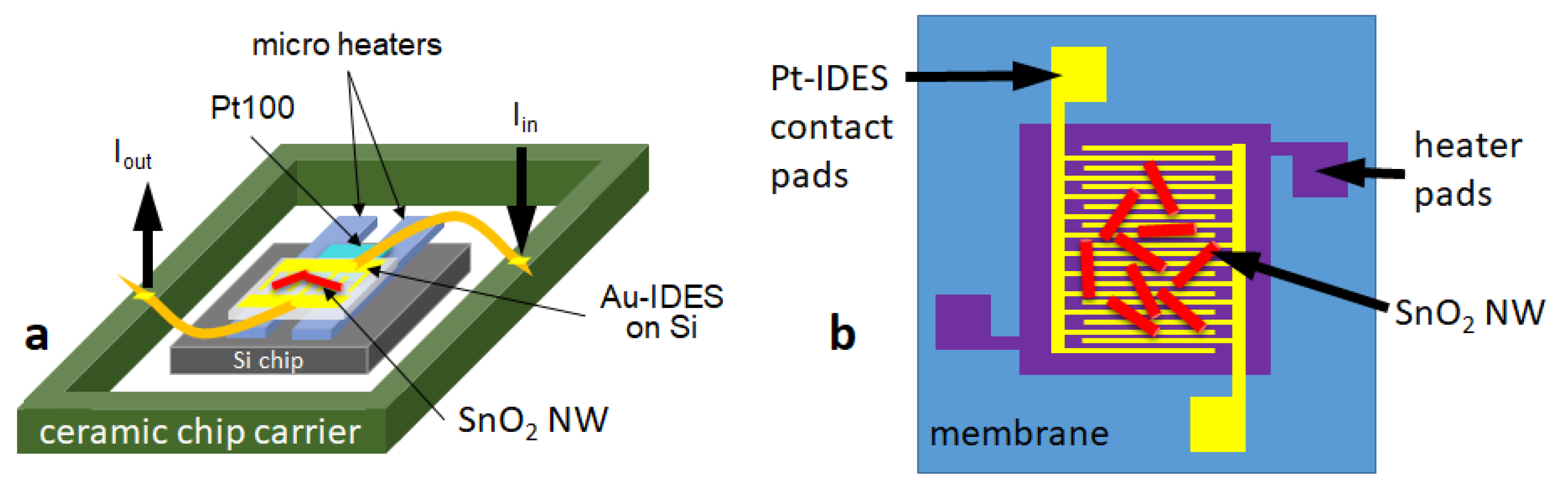
2.2. Transfer Printing Method and Device Fabrication
3. Results
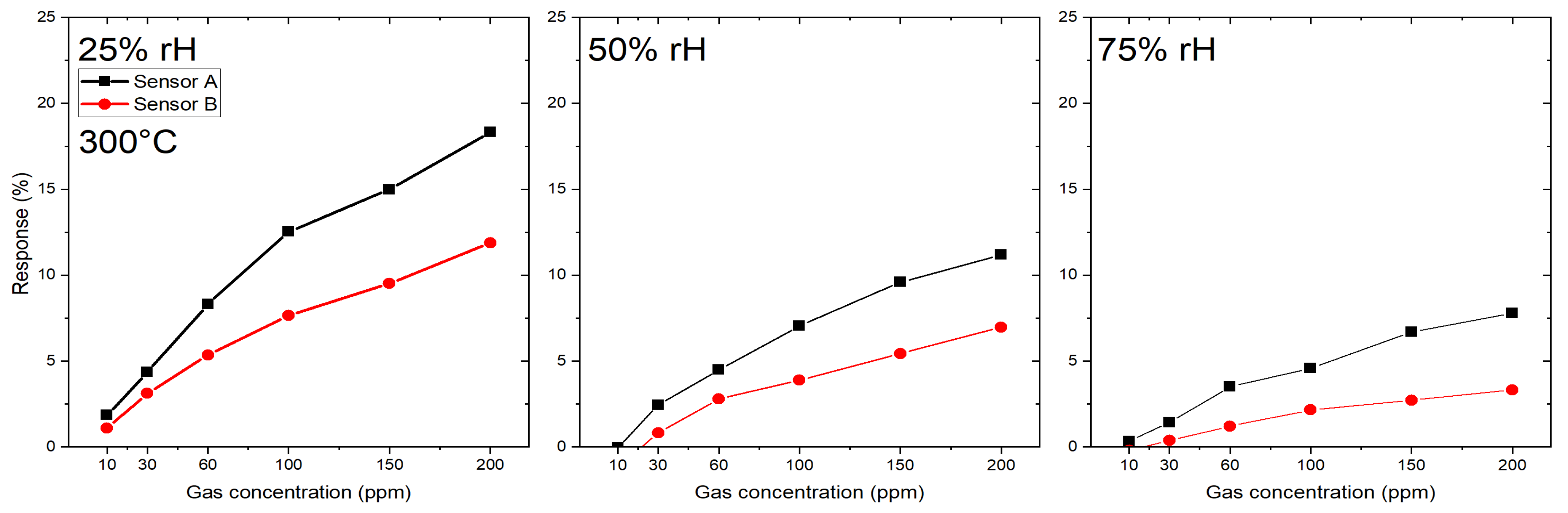
3.1. Gas Sensing Performance
3.1.1. Si-Based (SB) Gas Sensors
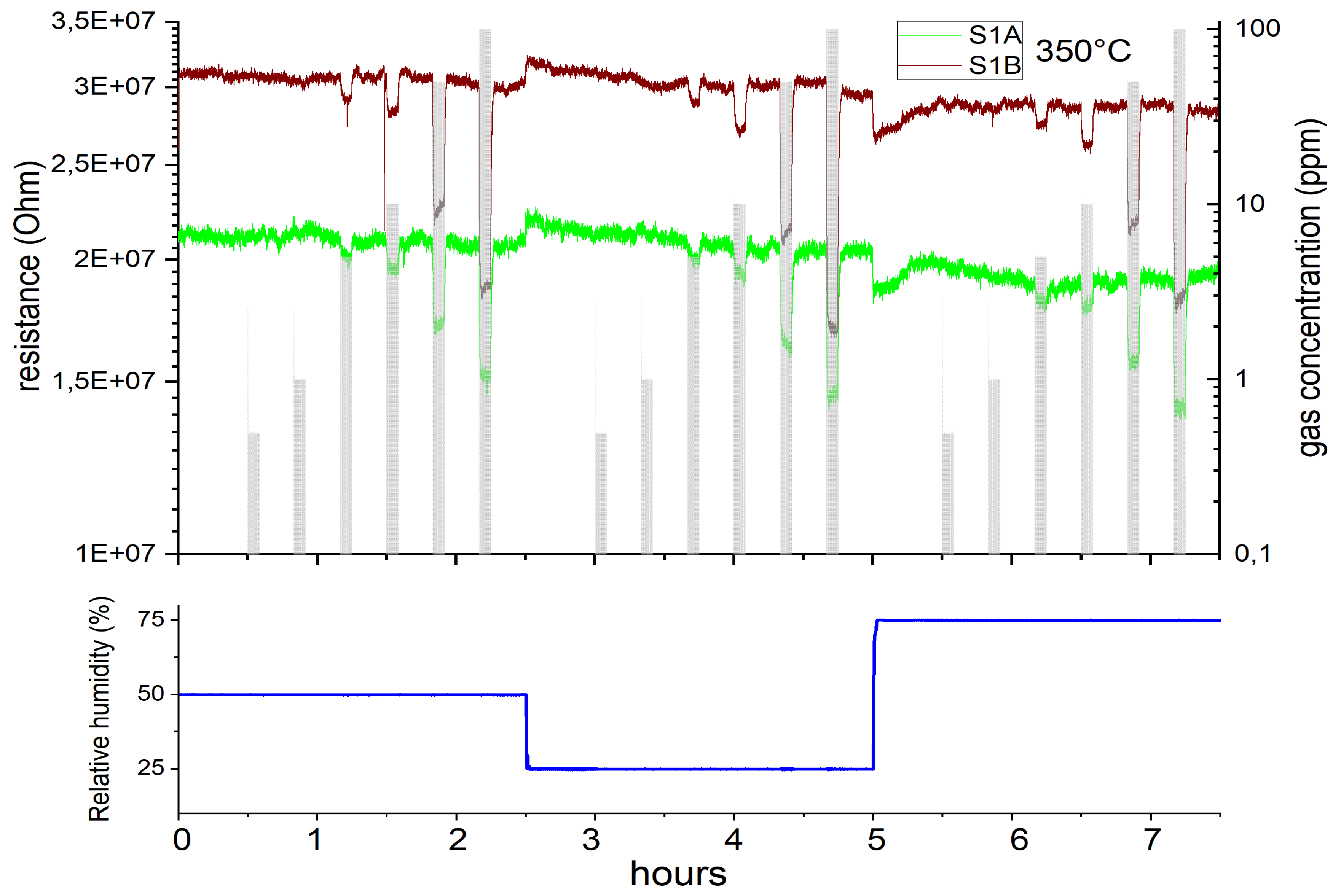
3.1.2. Microhotplate-Based Gas Sensors
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, Y.F.; Liu, S.B.; Meng, F.L.; Liu, J.Y.; Jin, Z.; Kong, L.T.; Liu, J.H. Metal oxide nanostructures and their gas sensing properties: A review. Sensors 2012, 12, 2610–2631. [Google Scholar] [CrossRef] [PubMed]
- Air Quality Deteriorating in Many of the World’s Cities; World Health Organization: Geneva, Switzerland, 2014.
- Korotcenkov, G. Metal oxides for solid-state gas sensors: What determines our choice? Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Comini, E. One-and two-dimensional metal oxide nanostructures for chemical sensing. In Semiconductor Gas Sensors; Elsevier: Amsterdam, The Netherlands, 2013; pp. 299–315. [Google Scholar]
- Barsan, N.; Koziej, D.; Weimar, U. Metal oxide-based gas sensor research: How to? Sens. Actuators B Chem. 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Ponzoni, A.; Baratto, C.; Cattabiani, N.; Falasconi, M.; Galstyan, V.; Nunez-Carmona, E.; Rigoni, F.; Sberveglieri, V.; Zambotti, G.; Zappa, D. Metal oxide gas sensors, a survey of selectivity issues addressed at the SENSOR Lab, Brescia (Italy). Sensors 2017, 17, 714. [Google Scholar] [CrossRef] [PubMed]
- Song, J.M.; Lee, J.S. Self-assembled nanostructured resistive switching memory devices fabricated by templated bottom-up growth. Sci. Rep. 2016, 6, 18967. [Google Scholar] [CrossRef] [PubMed]
- Gund, G.S.; Dubal, D.P.; Chodankar, N.R.; Cho, J.Y.; Gomez-Romero, P.; Park, C.; Lokhande, C.D. Low-cost flexible supercapacitors with high-energy density based on nanostructured MnO2 and Fe2O3 thin films directly fabricated onto stainless steel. Sci. Rep. 2015, 5, 12454. [Google Scholar] [CrossRef] [PubMed]
- Gedamu, D.; Paulowicz, I.; Kaps, S.; Lupan, O.; Wille, S.; Haidarschin, G.; Mishra, Y.K.; Adelung, R. Rapid fabrication technique for interpenetrated ZnO nanotetrapod networks for fast UV sensors. Adv. Mater. 2014, 26, 1541–1550. [Google Scholar] [CrossRef]
- Ahmad, R.; Tripathy, N.; Jang, N.K.; Khang, G.; Hahn, Y.B. Fabrication of highly sensitive uric acid biosensor based on directly grown ZnO nanosheets on electrode surface. Sens. Actuators B Chem. 2015, 206, 146–151. [Google Scholar] [CrossRef]
- Köck, A.; Tischner, A.; Maier, T.; Kast, M.; Edtmaier, C.; Gspan, C.; Kothleitner, G. Atmospheric pressure fabrication of SnO2-nanowires for highly sensitive CO and CH4 detection. Sens. Actuators B Chem. 2009, 138, 160–167. [Google Scholar] [CrossRef]
- Choudhury, S.; Betty, C.; Bhattacharyya, K.; Saxena, V.; Bhattacharya, D. Nanostructured PdO thin film from Langmuir–Blodgett precursor for room-temperature H2 gas sensing. ACS Appl. Mater. Interfaces 2016, 8, 16997–17003. [Google Scholar] [CrossRef]
- Sabet, M.; Salavati-Niasari, M. Deposition of lead sulfide nanostructure films on TiO2 surface via different chemical methods due to improving dye-sensitized solar cells efficiency. Electrochim. Acta 2015, 169, 168–179. [Google Scholar] [CrossRef]
- Yao, J.; Yan, H.; Lieber, C.M. A nanoscale combing technique for the large-scale assembly of highly aligned nanowires. Nat. Nanotechnol. 2013, 8, 329. [Google Scholar] [CrossRef] [PubMed]
- Noh, Y.Y.; Cheng, X.; Sirringhaus, H.; Sohn, J.I.; Welland, M.E.; Kang, D.J. Ink-jet printed ZnO nanowire field effect transistors. Appl. Phys. Lett. 2007, 91, 043109. [Google Scholar] [CrossRef]
- Yerushalmi, R.; Jacobson, Z.A.; Ho, J.C.; Fan, Z.; Javey, A. Large scale, highly ordered assembly of nanowire parallel arrays by differential roll printing. Appl. Phys. Lett. 2007, 91, 203104. [Google Scholar] [CrossRef]
- Javey, A.; Nam, S.; Friedman, R.S.; Yan, H.; Lieber, C.M. Layer-by-layer assembly of nanowires for three-dimensional, multifunctional electronics. Nano Lett. 2007, 7, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Meitl, M.A.; Zhu, Z.T.; Kumar, V.; Lee, K.J.; Feng, X.; Huang, Y.Y.; Adesida, I.; Nuzzo, R.G.; Rogers, J.A. Transfer printing by kinetic control of adhesion to an elastomeric stamp. Nat. Mater. 2006, 5, 33. [Google Scholar] [CrossRef]
- McAlpine, M.C.; Ahmad, H.; Wang, D.; Heath, J.R. Highly ordered nanowire arrays on plastic substrates for ultrasensitive flexible chemical sensors. Nat. Mater. 2007, 6, 379. [Google Scholar] [CrossRef]
- Liao, L.; Bai, J.; Lin, Y.C.; Qu, Y.; Huang, Y.; Duan, X. High-Performance Top-Gated Graphene-Nanoribbon Transistors Using Zirconium Oxide Nanowires as High-Dielectric-Constant Gate Dielectrics. Adv. Mater. 2010, 22, 1941–1945. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, D.R.; Zheng, X. Fabricating nanowire devices on diverse substrates by simple transfer-printing methods. Proc. Natl. Acad. Sci. USA 2010, 107, 9950–9955. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Ho, C.M.; Wong, T.S. Minimal size of coffee ring structure. J. Phys. Chem. B 2010, 114, 5269–5274. [Google Scholar] [CrossRef]
- Pregl, S. Fabrication and Characterization of a Silicon Nanowire Based Schottky-Barrier Field Effect Transistor Platform for Functional Electronics and Biosensor Applications. Ph.D. Thesis, Technische Universitat Dresden, Dresden, Germany, 2015. [Google Scholar]
- Deluca, M.; Wimmer-Teubenbacher, R.; Mitterhuber, L.; Mader, J.; Rohracher, K.; Holzer, M.; Köck, A. In-situ temperature measurement on CMOS integrated micro-hotplates for gas sensing devices. Sensors 2019, 19, 672. [Google Scholar] [CrossRef] [PubMed]
- Tulzer, G.; Baumgartner, S.; Brunet, E.; Mutinati, G.; Steinhauer, S.; Köck, A.; Barbano, P.; Heitzinger, C. Kinetic parameter estimation and fluctuation analysis of CO at SnO2 single nanowires. Nanotechnology 2013, 24, 315501. [Google Scholar] [CrossRef] [PubMed]
- Steinhauer, S.; Singh, V.; Cassidy, C.; Gspan, C.; Grogger, W.; Sowwan, M.; Köck, A. Single CuO nanowires decorated with size-selected Pd nanoparticles for CO sensing in humid atmosphere. Nanotechnology 2015, 26, 175502. [Google Scholar] [CrossRef] [PubMed]
- Gurlo, A. Nanosensors: Towards morphological control of gas sensing activity. SnO2, In2O3, ZnO and WO3 case studies. Nanoscale 2011, 3, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Tischner, A.; Maier, T.; Stepper, C.; Köck, A. Ultrathin SnO2 gas sensors fabricated by spray pyrolysis for the detection of humidity and carbon monoxide. Sens. Actuators B Chem. 2008, 134, 796–802. [Google Scholar] [CrossRef]
- Brunet, E.; Maier, T.; Mutinati, G.C.; Steinhauer, S.; Köck, A.; Gspan, C.; Grogger, W. Comparison of the gas sensing performance of SnO2 thin film and SnO2 nanowire sensors. Sens. Actuators B Chem. 2012, 165, 110–118. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sosada-Ludwikowska, F.; Wimmer-Teubenbacher, R.; Sagmeister, M.; Köck, A. Transfer Printing Technology as a Straightforward Method to Fabricate Chemical Sensors Based on Tin Dioxide Nanowires. Sensors 2019, 19, 3049. https://doi.org/10.3390/s19143049
Sosada-Ludwikowska F, Wimmer-Teubenbacher R, Sagmeister M, Köck A. Transfer Printing Technology as a Straightforward Method to Fabricate Chemical Sensors Based on Tin Dioxide Nanowires. Sensors. 2019; 19(14):3049. https://doi.org/10.3390/s19143049
Chicago/Turabian StyleSosada-Ludwikowska, Florentyna, Robert Wimmer-Teubenbacher, Martin Sagmeister, and Anton Köck. 2019. "Transfer Printing Technology as a Straightforward Method to Fabricate Chemical Sensors Based on Tin Dioxide Nanowires" Sensors 19, no. 14: 3049. https://doi.org/10.3390/s19143049
APA StyleSosada-Ludwikowska, F., Wimmer-Teubenbacher, R., Sagmeister, M., & Köck, A. (2019). Transfer Printing Technology as a Straightforward Method to Fabricate Chemical Sensors Based on Tin Dioxide Nanowires. Sensors, 19(14), 3049. https://doi.org/10.3390/s19143049




