Gas Sensing Properties of Mg-Incorporated Metal–Organic Frameworks
Abstract
:1. Introduction
2. Experimental
2.1. Chemical
2.2. Synthesis of Mg-MOFs
2.3. Materials Characterization
2.4. Gas Sensing Tests
3. Results and Discussion
3.1. Material Properties of Mg-MOFs
3.2. Gas Sensing Properties of Mg-MOFs
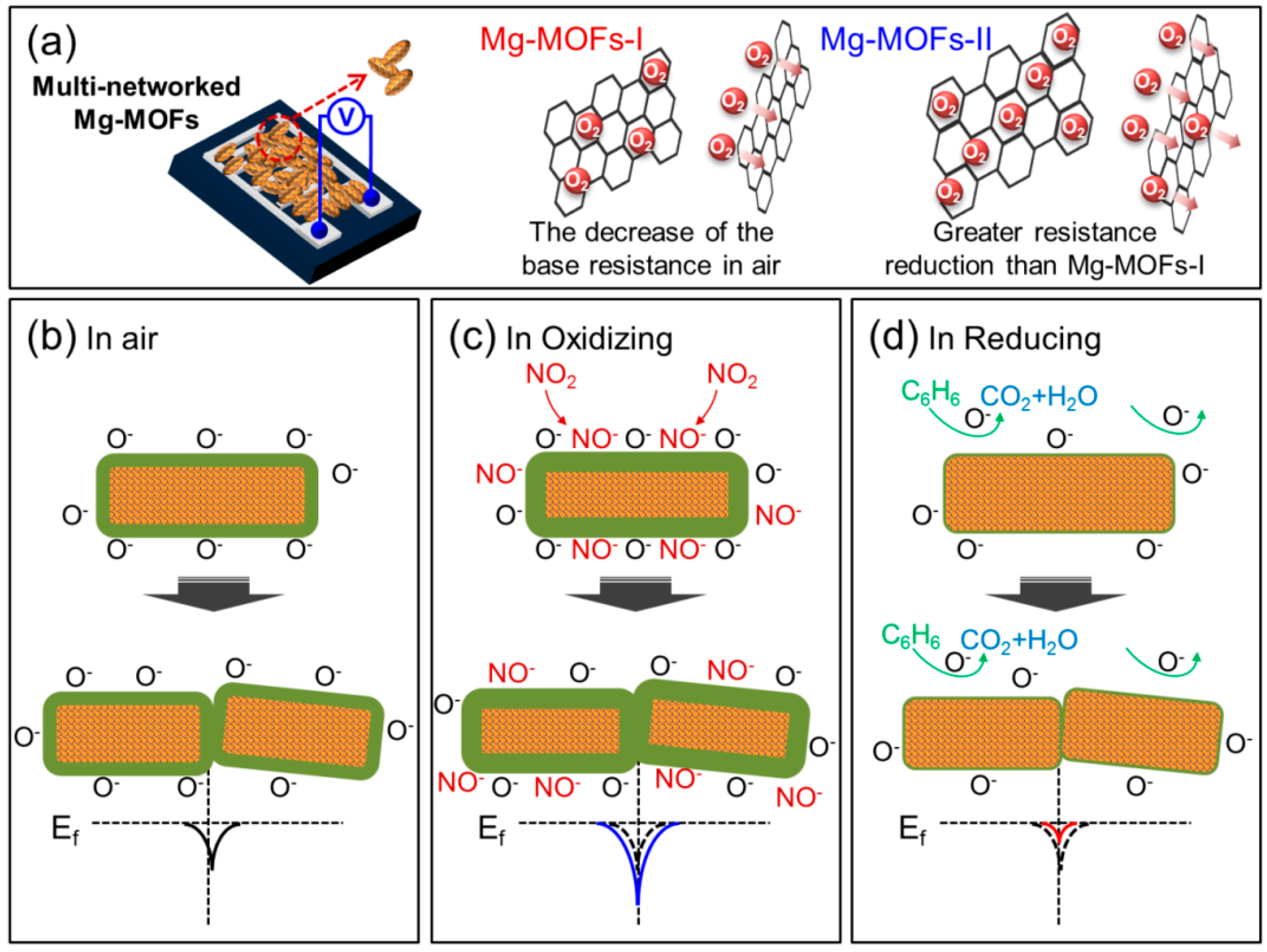
3.3. Gas Sensing Mechanism
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; van Duyne, R.P.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2011, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Suh, M.P.; Park, H.J.; Prasad, T.K.; Lim, D.W. Hydrogen storage in metal-organic frameworks. Chem. Rev. 2011, 112, 782–835. [Google Scholar] [CrossRef] [PubMed]
- Sadakiyo, M.; Yamada, T.; Kitagawa, H. Rational designs for highly proton-conductive metal-organic frameworks. J. Am. Chem. Soc. 2009, 131, 9906–9907. [Google Scholar] [CrossRef] [PubMed]
- Farrusseng, D.; Aguado, S.; Pinel, C. Metal-organic frameworks: Opportunities for catalysis. Angew. Chem. Int. Ed. 2009, 48, 7502–7513. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Serre, C.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Metal-organic frameworks as efficient materials for drug delivery. Angew. Chem. Int. Ed. 2006, 118, 6120–6124. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.H.; Long, J.R. Carbon dioxide capture in metal-organic frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef]
- Drobek, M.; Kim, J.-H.; Bechelany, M.; Vallicari, C.; Julbe, A.; Kim, S.S. MOF-based membrane encapsulated ZnO nanowires for enhanced gas sensor selectivity. ACS Appl. Mater. Interfaces 2016, 8, 8323–8328. [Google Scholar] [CrossRef]
- Li, T.; Wu, Y.; Huang, J.; Zhang, S. Gas sensors based on membrane diffusion for environmental monitoring. Sens. Actuators B 2017, 243, 566–578. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Campell, M.G.; Dincă, M. Electrically conductive porous metal-organic frameworks. Angew. Chem. Int. Ed. 2016, 55, 3566–3579. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Nguyen, H.L.; Nguyen, T.C.; Cordova, K.E.; Furukawa, H. High methanol uptake capacity in two new series of metal–organic frameworks: Promising materials for adsorption-driven heat pump applications. Chem. Mater. 2016, 28, 6243–6249. [Google Scholar] [CrossRef]
- Kim, J.-H.; Katoch, A.; Kim, S.-H.; Kim, S.S. Chemiresistive sensing behavior of SnO2 (n)-Cu2O (p) core-shell nanowires. ACS Appl. Mater. Interfaces 2015, 7, 15351–15358. [Google Scholar] [CrossRef]
- Choi, S.-W.; Katoch, A.; Kim, J.-H.; Kim, S.S. Striking sensing improvement of n-type oxide nanowires by electronic sensitization based on work function difference. J. Mater. Chem. C 2015, 3, 1521–1527. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, J.-Y.; Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Synthesis and gas sensing properties of membrane template-grown hollow ZnO nanowires. Nano Convergence 2017, 4, 27. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Low power-consumption CO gas sensors based on Au-functionalized SnO2-ZnO core-shell nanowires. Sens. Actuators B 2018, 267, 597–607. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Šutka, A.; Kodu, M.; Pärna, R.; Saar, R.; Juhnevica, I.; Jaaniso, R.; Kisand, V. Orthorhombic CaFe2O4: A promising p-type gas sensor. Sens. Actuators B 2016, 224, 260–265. [Google Scholar] [CrossRef]
- Deng, S.; Liu, X.; Chen, N.; Deng, D.; Xiao, X.; Wang, Y. A highly sensitive VOC gas sensor using p-type mesoporous Co3O4 nanosheets prepared by a facile chemical coprecipitation method. Sens. Actuators B 2016, 233, 615–623. [Google Scholar] [CrossRef]
- Dong, G.; Fan, H.; Tian, H.; Fang, J.; Li, Q. Gas-sensing and electrical properties of perovskite structure p-type barium-substituted bismuth ferrite. RSC Adv. 2015, 5, 29618–29623. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, M.; Mu, X.; Sen, S.; Insley, T.; Mason, A.J.; Kral, P.; Zeng, X. Highly sensitive capacitive gas sensing at ionic liquid-electrode interfaces. Anal. Chem. 2016, 88, 1959–1964. [Google Scholar] [CrossRef]
- Drobek, M.; Kim, J.-H.; Bechelany, M.; Vallicari, C.; Leroy, E.; Julbe, A.; Kim, S.S. Design and fabrication of highly selective H2 sensors based on SIM-1 nanomembrane-coated ZnO nanowires. Sens. Actuators B 2018, 264, 410–418. [Google Scholar] [CrossRef]








| Mg-MOFs-I | ||||||||||
| NO2 | O2 | H2S | H2 | C6H6 | ||||||
| Res. time (s) | Rec. time (s) | Res. time (s) | Rec. time (s) | Res. time (s) | Rec. time (s) | Res. time (s) | Rec. time (s) | Res. time (s) | Rec. time (s) | |
| 1 ppm | 124 | 221 | 477 | 590 | 482 | 265 | 359 | 216 | 251 | 855 |
| 5 ppm | 80 | 85 | 379 | 461 | 255 | 935 | 36 | 42 | 150 | 123 |
| 10 ppm | 121 | 182 | 233 | 447 | 178 | 303 | 10 | 59 | 460 | 331 |
| Mg-MOFs-II | ||||||||||
| NO2 | O2 | H2S | H2 | C6H6 | ||||||
| Res. time (s) | Rec. time (s) | Res. time (s) | Rec. time (s) | Res. time (s) | Rec. time (s) | Res. time (s) | Rec. time (s) | Res. time (s) | Rec. time (s) | |
| 1 ppm | 184 | 170 | 105 | 119 | 297 | 189 | 307 | 294 | 306 | 231 |
| 5 ppm | 227 | 129 | 195 | 119 | 278 | 197 | 325 | 227 | 307 | 165 |
| 10 ppm | 167 | 92 | 194 | 94 | 259 | 163 | 321 | 198 | 261 | 175 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Nguyen, T.-B.; Nguyen, D.-K.; Kim, J.-H.; Kim, J.-Y.; Phan, B.T.; Kim, S.S. Gas Sensing Properties of Mg-Incorporated Metal–Organic Frameworks. Sensors 2019, 19, 3323. https://doi.org/10.3390/s19153323
Lee J-H, Nguyen T-B, Nguyen D-K, Kim J-H, Kim J-Y, Phan BT, Kim SS. Gas Sensing Properties of Mg-Incorporated Metal–Organic Frameworks. Sensors. 2019; 19(15):3323. https://doi.org/10.3390/s19153323
Chicago/Turabian StyleLee, Jae-Hyoung, Thanh-Binh Nguyen, Duy-Khoi Nguyen, Jae-Hun Kim, Jin-Young Kim, Bach Thang Phan, and Sang Sub Kim. 2019. "Gas Sensing Properties of Mg-Incorporated Metal–Organic Frameworks" Sensors 19, no. 15: 3323. https://doi.org/10.3390/s19153323






