Obliquely Deposited Titanium Nitride Nanorod Arrays as Surface-Enhanced Raman Scattering Substrates †
Abstract
:1. Introduction
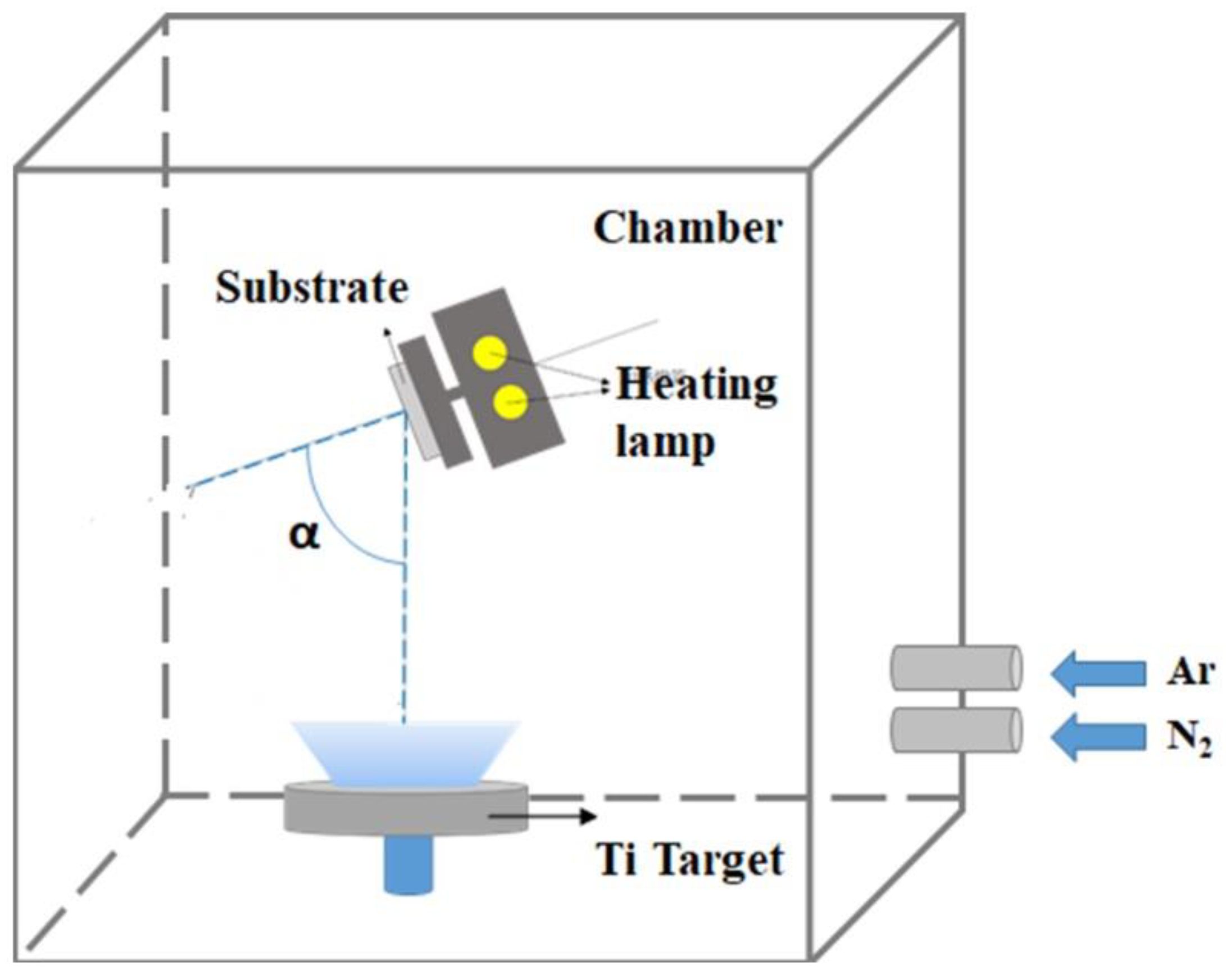
2. Experimental Setup and Methodology
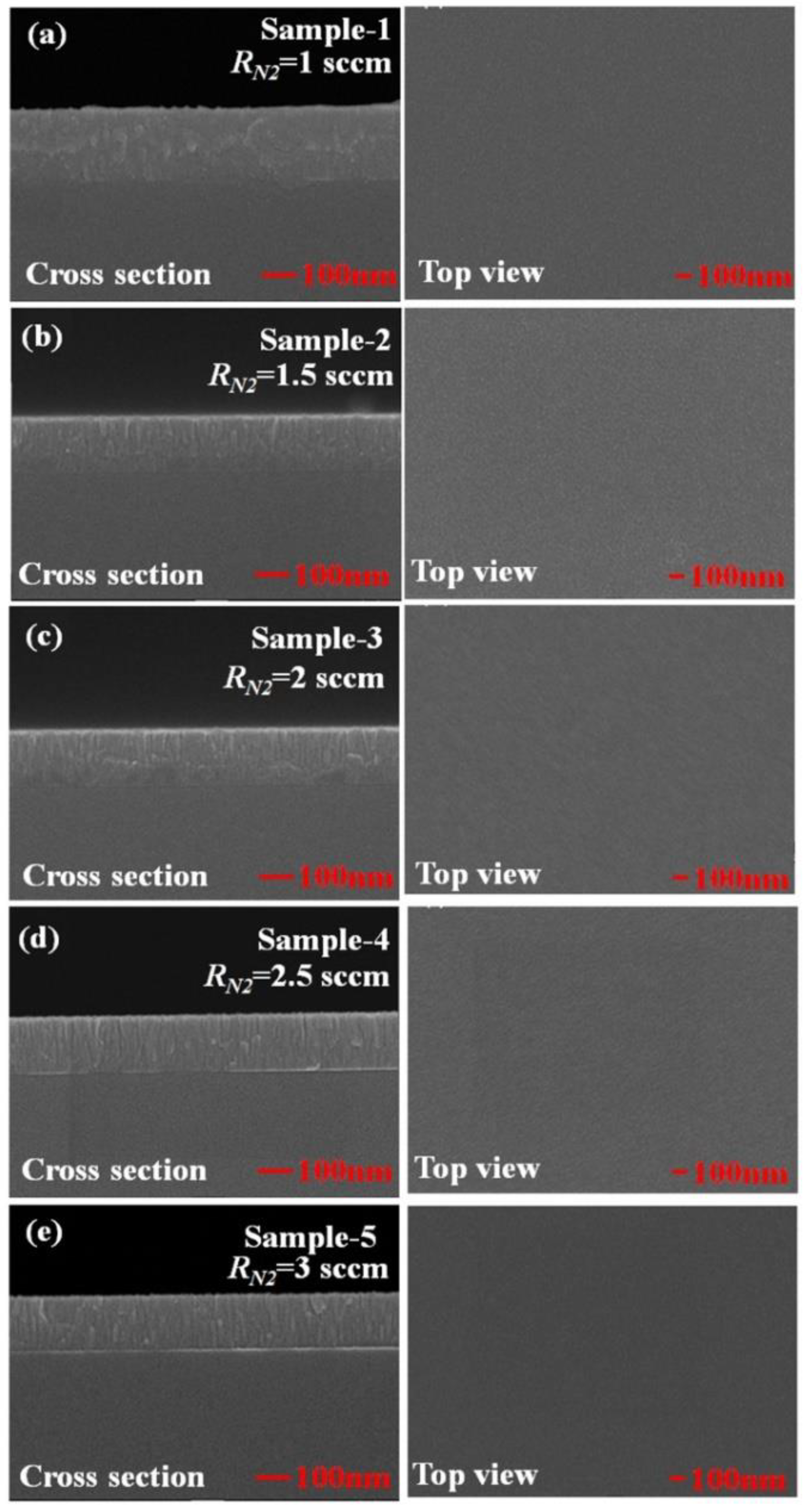
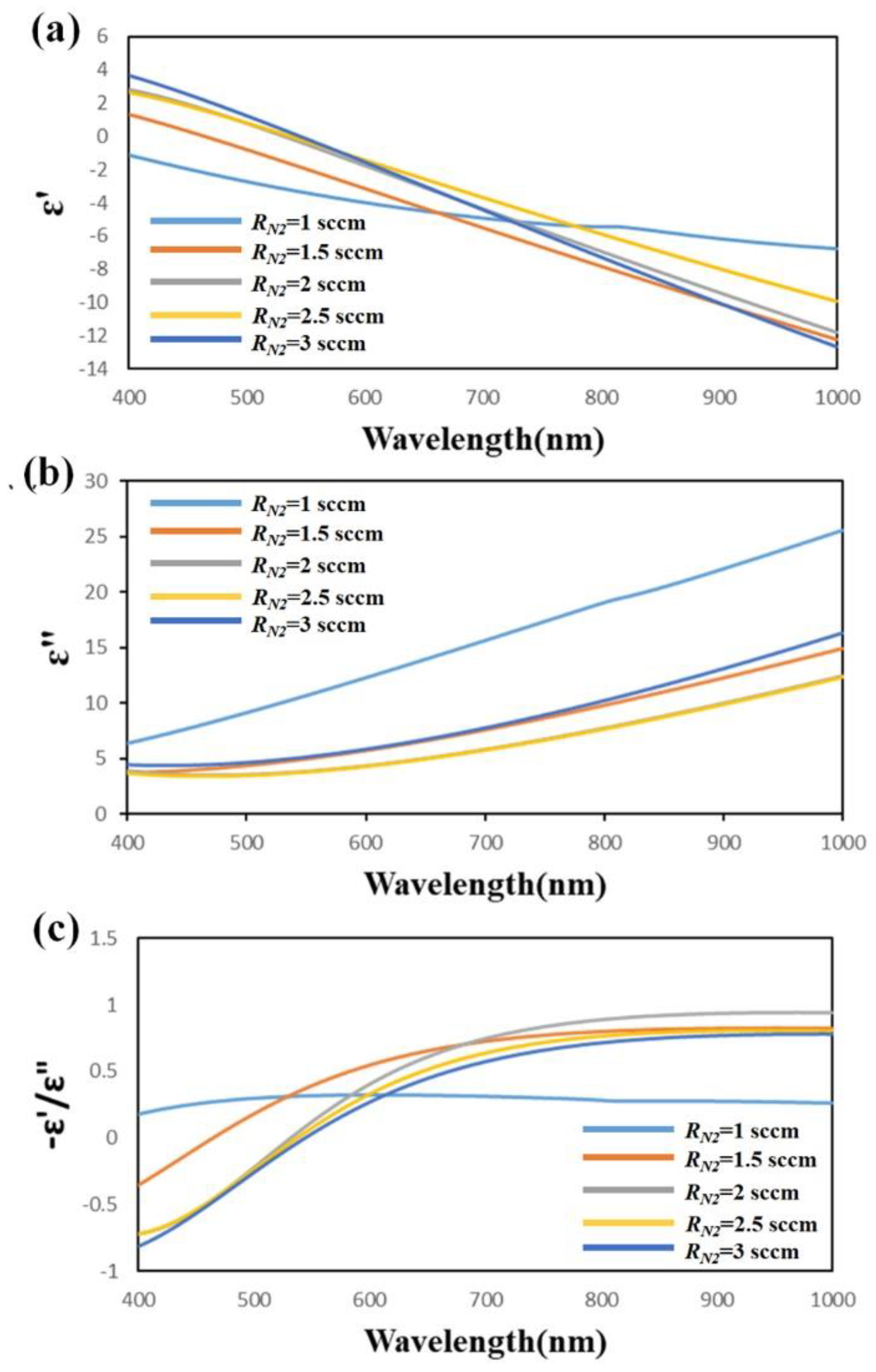
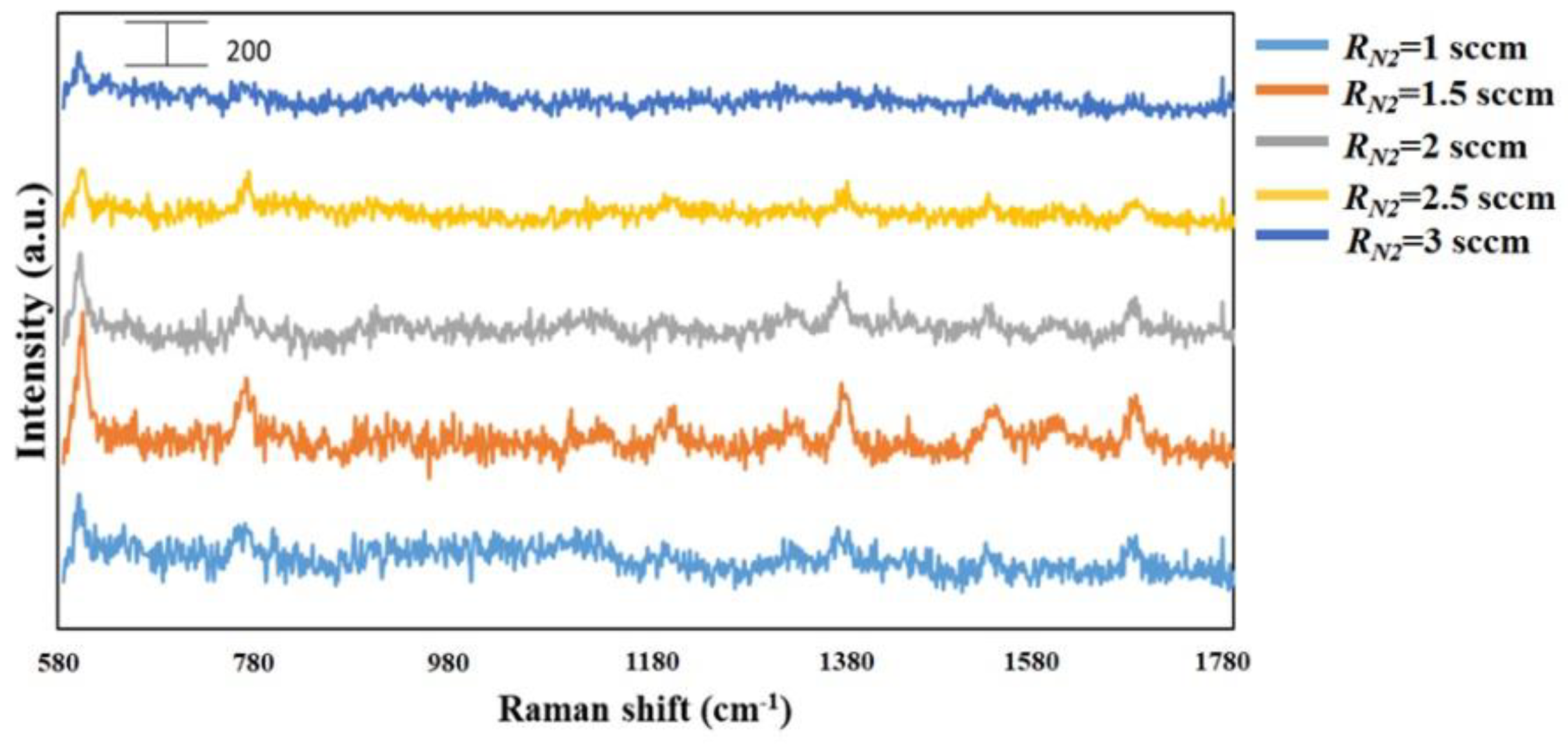
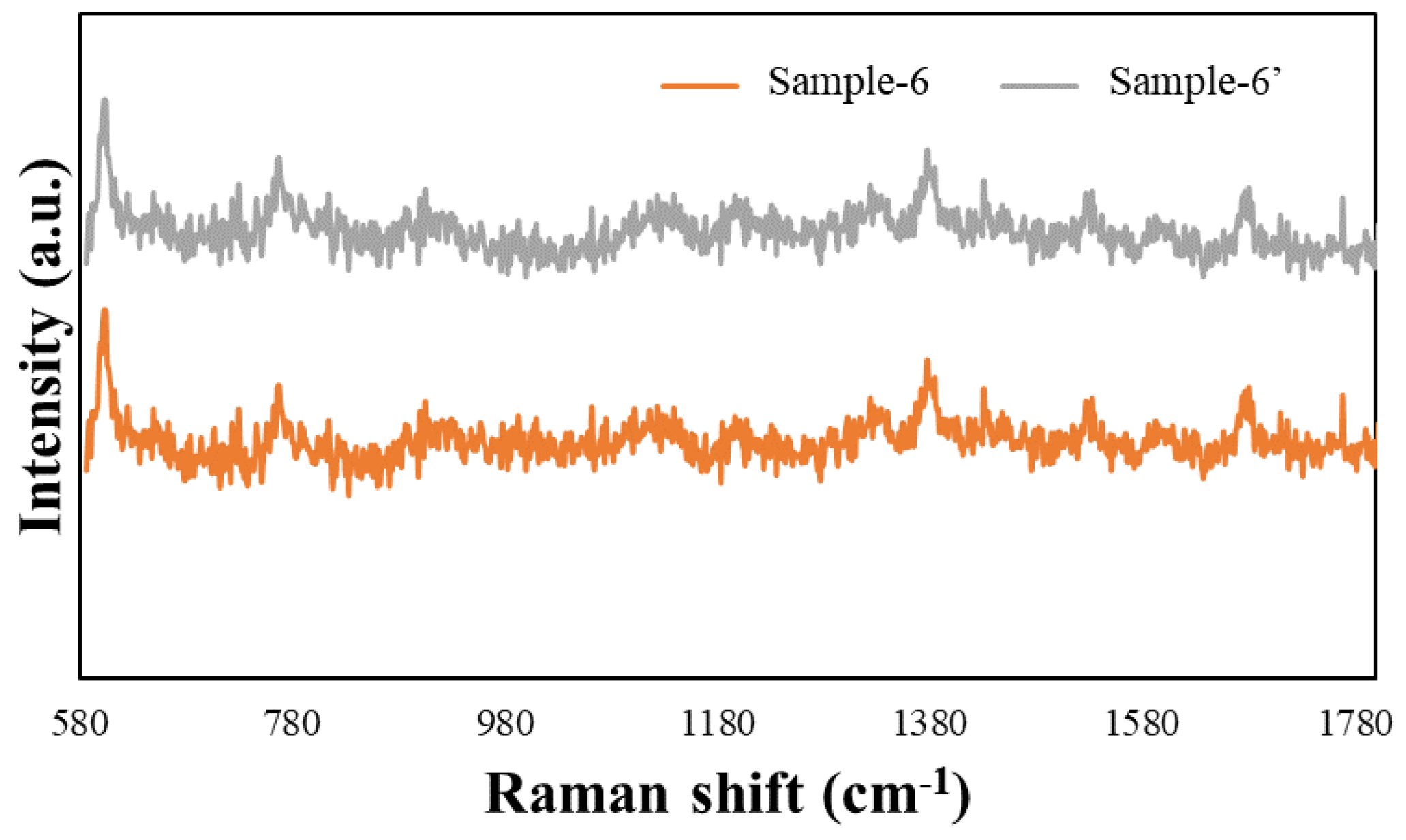
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stewart, M.E.; Anderton, C.R.; Thompson, L.B.; Maria, J.; Gray, S.K.; Rogers, J.A.; Nuzzo, R.G. Nuzzo, Nanostructured plasmonic Sensors. Chem. Rev. 2008, 108, 494. [Google Scholar] [CrossRef] [PubMed]
- Challener, W.A.; Peng, C.; Itagi, A.V.; Karns, D.; Peng, W.; Peng, Y.; Yang, X.; Zhu, X.; Gokemeijer, N.J.; Hsia, Y.T.; et al. Heat-assisted magnetic recording by a near-field transducer with efficient optical energy transfer. Nat. Photonics 2009, 3, 220–224. [Google Scholar] [CrossRef]
- Boltasseva, A.; Nikolajsen, T.; Leosson, K.; Kjaer, K.; Larsen, M.; Bozhevolnyi, S. Integrated optical components utilizing long-range surface plasmon polaritons. J. Light. Technol. 2005, 23, 413–422. [Google Scholar] [CrossRef]
- Campion, A.; Kambhampati, P. Surface-enhanced Raman scattering. Chem. Soc. Rev. 1998, 27, 241. [Google Scholar] [CrossRef]
- Moskovits, M. Surface-enhanced Raman spectroscopy: A brief retrospective. J. Raman Spectrosc. 2005, 36, 485–496. [Google Scholar] [CrossRef]
- Song, C.; Wang, Z.; Zhang, R.; Yang, J.; Tan, X.; Cui, Y. Highly sensitive immunoassay based on Raman reporter-labeled immune-Au aggregates and SERS-active immune substrate. Biosens. Bioelectron. 2009, 25, 826. [Google Scholar] [CrossRef]
- Bell, S.E.J.; Sirimuthu, N.M.S. Surface-Enhanced Raman Spectroscopy (SERS) for Sub-Micromolar Detection of DNA/RNA Mononucleotides. J. Am. Chem. Soc. 2006, 128, 15580–15581. [Google Scholar] [CrossRef]
- Chen, J.; Park, B.; Huang, Y.W.; Zhao, Y.; Kwon, Y. Label-free SERS detection of Salmonella Typhimurium on DNA aptamer modified AgNR substrates. J. Food Meas. Charact. 2017, 11, 1773–1779. [Google Scholar] [CrossRef]
- Langer, J.; De Aberasturi, D.J.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2019. [Google Scholar] [CrossRef]
- Benz, F.; Tserkezis, C.; Herrmann, L.O.; De Nijs, B.; Sanders, A.; Sigle, D.O.; Pukenas, L.; Evans, S.D.; Aizpurua, J.; Baumberg, J.J. Nanooptics of MolecularShunted Plasmonic Nanojunctions. Nano Lett. 2015, 15, 669–674. [Google Scholar] [CrossRef]
- Baumberg, J.J.; Aizpurua, J.; Mikkelsen, M.H.; Smith, D.R. Extreme nanophotonics from ultrathin metallic gaps. Nat. Mater. 2019, 18, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.; Hamidi, S.M. Bi:YIG@Au magneto-plasmonic core-shell nano-grating with robust, high magneto-optical figure of merit. J. Magn. Magn. Mater. 2019, 493, 165709. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Chaney, S.B.; Zhang, Z.Y. Absorbance spectra of aligned Ag nanorod arrays prepared by oblique angle deposition. J. Appl. Phys. 2006, 100, 63527. [Google Scholar] [CrossRef]
- Jen, Y.J.; Liu, W.C.; Hsiao, C.Y.; Lin, P.C.; Yu, C.L.; Chan, T.L. Obliquely Deposited Gold Nanohelices on Lithography-Free Prepared Nanoseeded Surfaces. Nanoscale Res. Lett. 2017, 12, 485. [Google Scholar] [CrossRef]
- Jen, Y.J.; Weng Lin, Y.; Huang, Y.J.; Chan, T.L. The Effect of Glancing Angle Deposition Conditions on the Morphology of a Silver Nanohelix Array. Coatings 2017, 7, 140. [Google Scholar] [CrossRef]
- Liu, Y.J.; Zhang, Z.Y.; Zhao, Q.; Dluhy, R.A.; Zhao, Y.P. Surface Enhanced Raman Scattering from an Ag Nanorod Array Substrate: The Site Dependent Enhancement and Layer Absorbance Effect. J. Phys. Chem. C 2009, 113, 9664–9669. [Google Scholar] [CrossRef]
- He, L.; Han, S.T.; He, Y.; Lee, S.T.; Huang, J.; Xu, T.; Chen, L.; Zhang, K. Silver nanosheet-coated inverse opal film as a highly active and uniform SERS substrate. J. Mater. Chem. 2012, 22, 1370–1374. [Google Scholar] [CrossRef]
- Jung, G.B.; Bae, Y.M.; Lee, Y.J.; Ryu, S.H.; Park, H.K. Nanoplasmonic Au nanodot arrays as an SERS substrate for biomedical applications. Appl. Surf. Sci. 2013, 282, 161–164. [Google Scholar] [CrossRef]
- Jen, Y.J.; Chan, S.; Huang, J.W.; Jheng, C.Y.; Liu, W.C. Self-Shadowing deposited pure metal nanohelix arrays and SERS application. Nanoscale Res. Lett. 2015, 10, 498. [Google Scholar] [CrossRef]
- Musumeci, A.; Gosztola, D.; Schiller, T.; Dimitrijevic, N.M.; Mujica, V.; Martin, D.; Rajh, T. SERS of Semiconducting Nanoparticles (TiO2Hybrid Composites). J. Am. Chem. Soc. 2009, 131, 6040–6041. [Google Scholar] [CrossRef]
- Wang, X.; She, G.; Xu, H.; Mu, L.; Shi, W. The surface-enhanced Raman scattering from ZnO nanorod arrays and its application for chemosensors. Sens. Actuators B Chem. 2014, 193, 745–751. [Google Scholar] [CrossRef]
- Naik, G.V.; Schroeder, J.L.; Ni, X.; Kildishev, A.V.; Sands, T.D.; Boltasseva, A. Titanium nitride as a plasmonic material for visible and near-infrared wavelengths. Opt. Mater. Express. 2012, 2, 478. [Google Scholar] [CrossRef]
- Boltasseva, A.; Atwater, H.A. Low-loss plasmonic metamaterials. Science 2011, 331, 290. [Google Scholar] [CrossRef]
- Guler, U.; Boltasseva, A.; Shalaev, V.M. Refractory plasmonics. Science 2014, 344, 263. [Google Scholar] [CrossRef]
- Braic, L.; Vasilantonakis, N.; Mihai, A.; Garcia, I.J.V.; Fearn, S.; Zou, B.; Alford, N.M.; Doiron, B.; Oulton, R.F.; Maier, S.A.; et al. Titanium Oxynitride Thin Films with Tunable Double Epsilon-Near-Zero Behavior for Nanophotonic Applications. ACS Appl. Mater. Interfaces 2017, 9, 29857–29862. [Google Scholar] [CrossRef]
- Kaisar, N.; Huang, Y.T.; Jou, S.; Kuo, H.F.; Huang, B.R.; Chen, C.C.; Hsieh, Y.F.; Chung, Y.C. Surface-enhanced Raman scattering substrates of flat and wrinkly titanium nitride thin films by sputter deposition. Surf. Coat. Technol. 2018, 337, 434–438. [Google Scholar] [CrossRef]
- Boldyš, J.; Hrach, R. Multiscale semicontinuous thin film descriptors. Cent. Eur. J. Phys. 2004, 2, 645–659. [Google Scholar] [CrossRef]
- Sit, J.C.; Robbie, K.; Brett, M.J. Advanced techniques for glancing angle deposition. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 1998, 16, 1115. [Google Scholar]
- Jen, Y.J.; Lakhtakia, A.; Yu, C.W.; Jhou, J.J.; Wang, W.H.; Lin, M.J.; Wu, H.M.; Liao, H.S. Silver/silicon dioxide/silver sandwich films in the blue-to-red spectral regime with negative-real refractive index. Appl. Phys. Lett. 2011, 99, 181117. [Google Scholar] [CrossRef]
- Shah, D.; Reddy, H.; Kinsey, N.; Shalaev, V.M.; Boltasseva, A. Optical Properties of Plasmonic Ultrathin TiN Films. Adv. Opt. Mater. 2017, 5, 1700065. [Google Scholar] [CrossRef]
- Lalisse, A.; Tessier, G.; Plain, J.; Baffou, G. Plasmonic efficiencies of nanoparticles made of metal nitrides (TiN, ZrN) compared with gold. Sci. Rep. 2016, 6, 38647. [Google Scholar] [CrossRef]
- Ponon, N.K.; Appleby, D.J.; Arac, E.; King, P.; Ganti, S.; Kwa, K.S.; O’Neill, A.; O’Neill, A. Effect of deposition conditions and post deposition anneal on reactively sputtered titanium nitride thin films. Thin Solid Films 2015, 578, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Yan, B.; Wong, S.M.; Li, X.; Zhou, W.; Yu, T.; Shen, Z.; Yu, H.; Fan, H.J. Fabrication and SERS Performance of Silver-Nanoparticle-Decorated Si/ZnO Nanotrees in Ordered Arrays. ACS Appl. Mater. Interfaces 2010, 2, 1824–1828. [Google Scholar] [CrossRef]
- El-Abassy, R.M.; Donfack, P.; Materny, A. Visible Raman spectroscopy for the discrimination of olive oils from different vegetable oils and the detection of adulteration. J. Raman Spectrosc. 2009, 40, 1284–1289. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Blackie, E.; Meyer, M.; Etchegoin, P.G. Surface Enhanced Raman Scattering Enhancement Factors: A Comprehensive Study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Dong, Z.; Wei, H.; Chen, Y.; Wang, R.; Zhao, J.; Lin, J.; Bu, J.; Wei, Y.; Cui, Y.; Yu, Y. Surface enhanced Raman scattering activity of TiN thin film prepared via nitridation of sol-gel derived TiO2 film. AOPC 2015 Adv. Disp. Technol. Micro/Nano Opt. Imaging Technol. Appl. 2015, 9672, 967209. [Google Scholar]
- Zhao, J.; Lin, J.; Wei, H.; Li, X.; Zhang, W.; Zhao, G.; Bu, J.; Chen, Y. Surface enhanced Raman scattering substrates based on titanium nitride nanorods. Opt. Mater. 2015, 47, 219–224. [Google Scholar] [CrossRef]
- Dick, B.; Brett, M.J.; Smy, T.; Belov, M.; Freeman, M.R. Periodic submicrometer structures by sputtering. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2001, 19, 1813. [Google Scholar] [CrossRef]
- Naik, G.V.; Shalaev, V.M.; Boltasseva, A. Alternative plasmonic materials: Beyond gold and silver. Adv. Mater. 2013, 25, 3264–3294. [Google Scholar] [CrossRef]
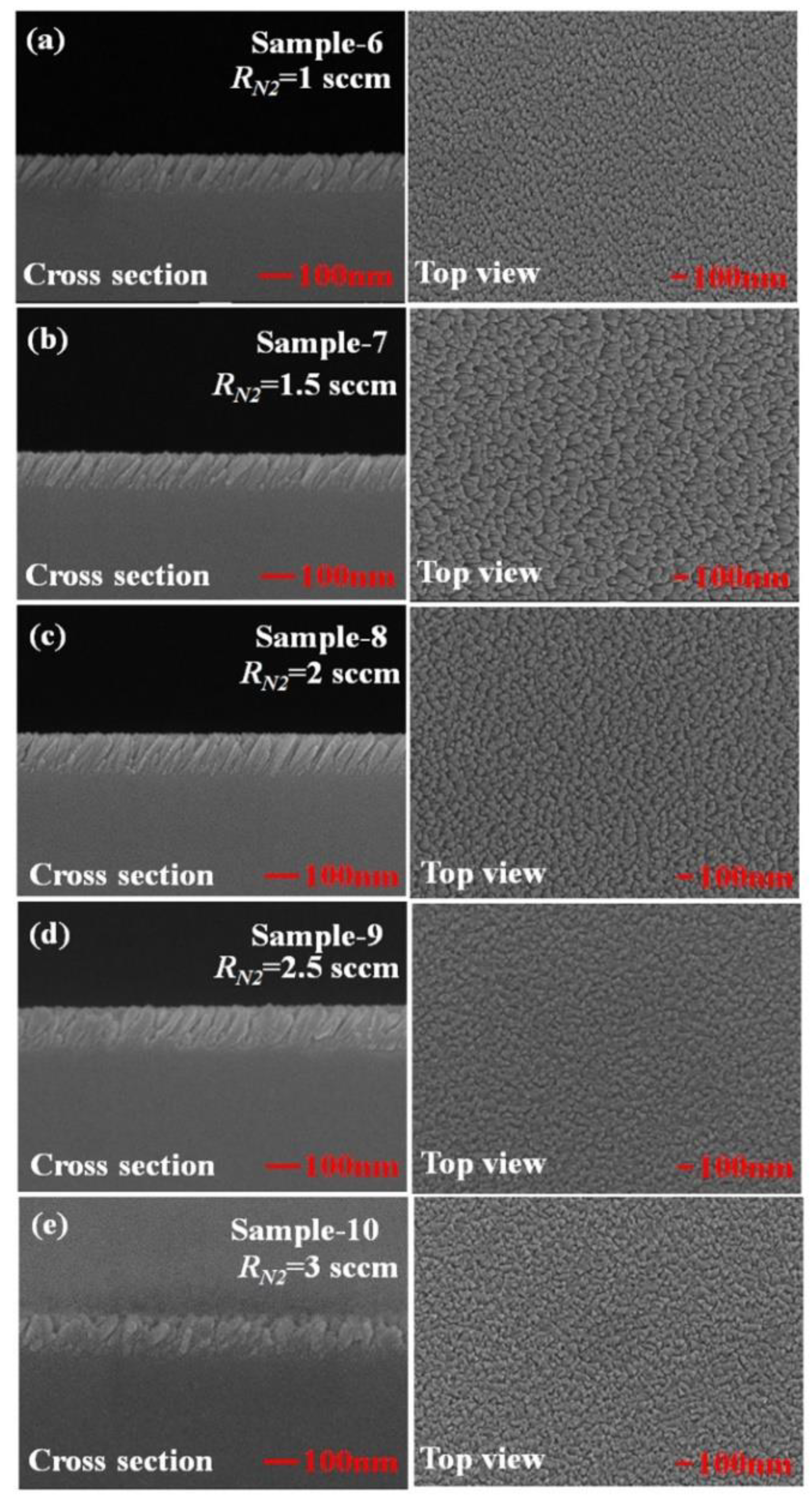






| β (°) | w (nm) | d (nm) | p (nm) | P (%) | |
|---|---|---|---|---|---|
| Sample-6 | 39 ± 5 | 30 ± 5 | 117 ± 10 | 21 ± 5 | 7.4 |
| Sample-7 | 32 ± 5 | 26 ± 5 | 112 ± 15 | 30 ± 5 | 10.3 |
| Sample-8 | 25 ± 5 | 25 ± 5 | 123 ± 15 | 21 ± 5 | 8.3 |
| Sample-9 | 31 ± 5 | 28 ± 5 | 130 ± 8 | 22 ± 5 | 9.5 |
| Sample-10 | 37 ± 5 | 36 ± 5 | 134 ± 6 | 20 ± 5 | 8.9 |
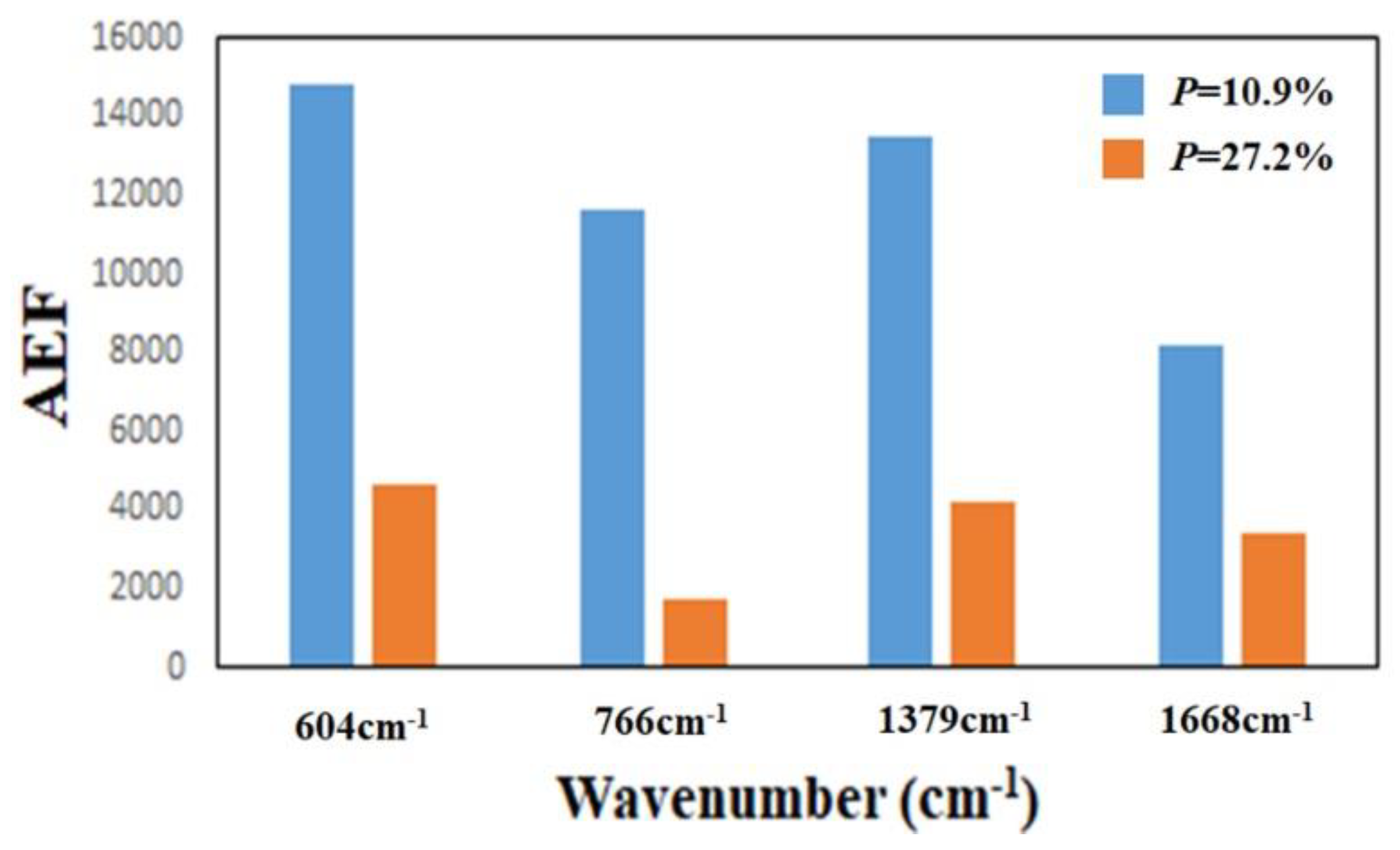
| Raman Peak (cm−1) | Sample-6 AEF | Sample-7 AEF | Sample-8 AEF | Sample-9 AEF | Sample-10 AEF |
|---|---|---|---|---|---|
| 604 | 8.39 × 103 | 1.34 × 104 | 7.59 × 103 | 6.53 × 103 | 4.98 × 103 |
| 766 | 3.01 × 103 | 1.15 × 104 | 2.42 × 103 | 4.05 × 103 | 3.60 × 103 |
| 1379 | 4.94 × 103 | 1.09 × 104 | 4.47 × 103 | 5.08 × 103 | 4.81 × 103 |
| 1668 | 3.70 × 103 | 6.26 × 103 | 1.90 × 103 | 2.09 × 103 | 3.90 × 103 |
| β (°) | w (nm) | d (nm) | p (nm) | P (%) | |
|---|---|---|---|---|---|
| Sample-11 | 31 ± 5 | 26 ± 5 | 110 ± 5 | 30 ± 5 | 9.3 |
| Sample-12 | 32 ± 5 | 33 ± 3 | 183 ± 10 | 34 ± 5 | 10.9 |
| Sample-13 | 35 ± 5 | 58 ± 5 | 297 ± 15 | 71 ± 15 | 17.5 |
| Sample-14 | 40 ± 3 | 48 ± 5 | 246 ± 15 | 159 ± 20 | 27.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jen, Y.-J.; Lin, M.-J.; Cheang, H.-L.; Chan, T.-L. Obliquely Deposited Titanium Nitride Nanorod Arrays as Surface-Enhanced Raman Scattering Substrates. Sensors 2019, 19, 4765. https://doi.org/10.3390/s19214765
Jen Y-J, Lin M-J, Cheang H-L, Chan T-L. Obliquely Deposited Titanium Nitride Nanorod Arrays as Surface-Enhanced Raman Scattering Substrates. Sensors. 2019; 19(21):4765. https://doi.org/10.3390/s19214765
Chicago/Turabian StyleJen, Yi-Jun, Meng-Jie Lin, Hou-Lon Cheang, and Teh-Li Chan. 2019. "Obliquely Deposited Titanium Nitride Nanorod Arrays as Surface-Enhanced Raman Scattering Substrates" Sensors 19, no. 21: 4765. https://doi.org/10.3390/s19214765





