Design and Analyses of a Transdermal Drug Delivery Device (TD3) †
Abstract
:1. Introduction
2. Transdermal Drug Delivery Device (TD3)
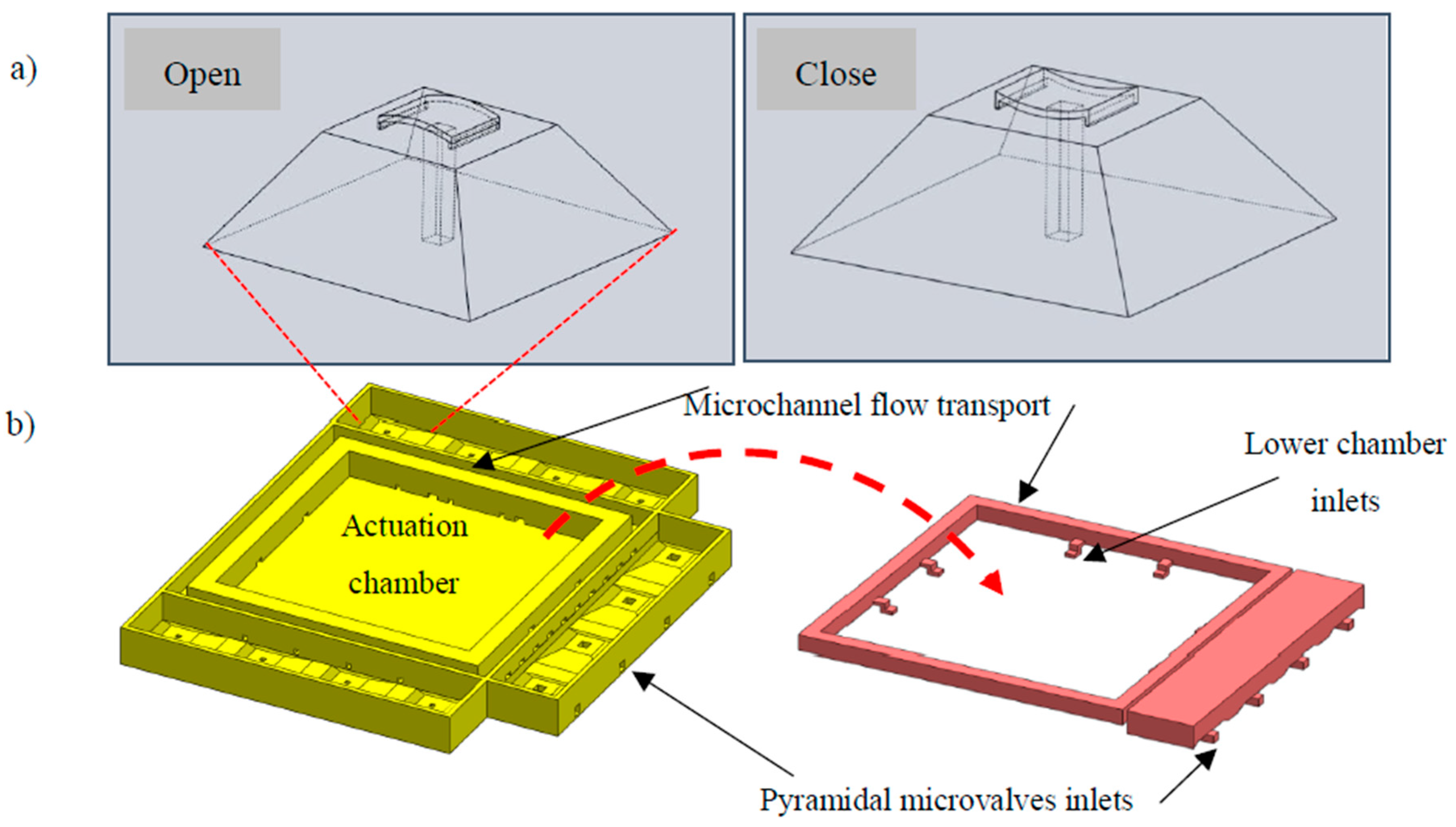
2.1. TD3 Actuation Principle
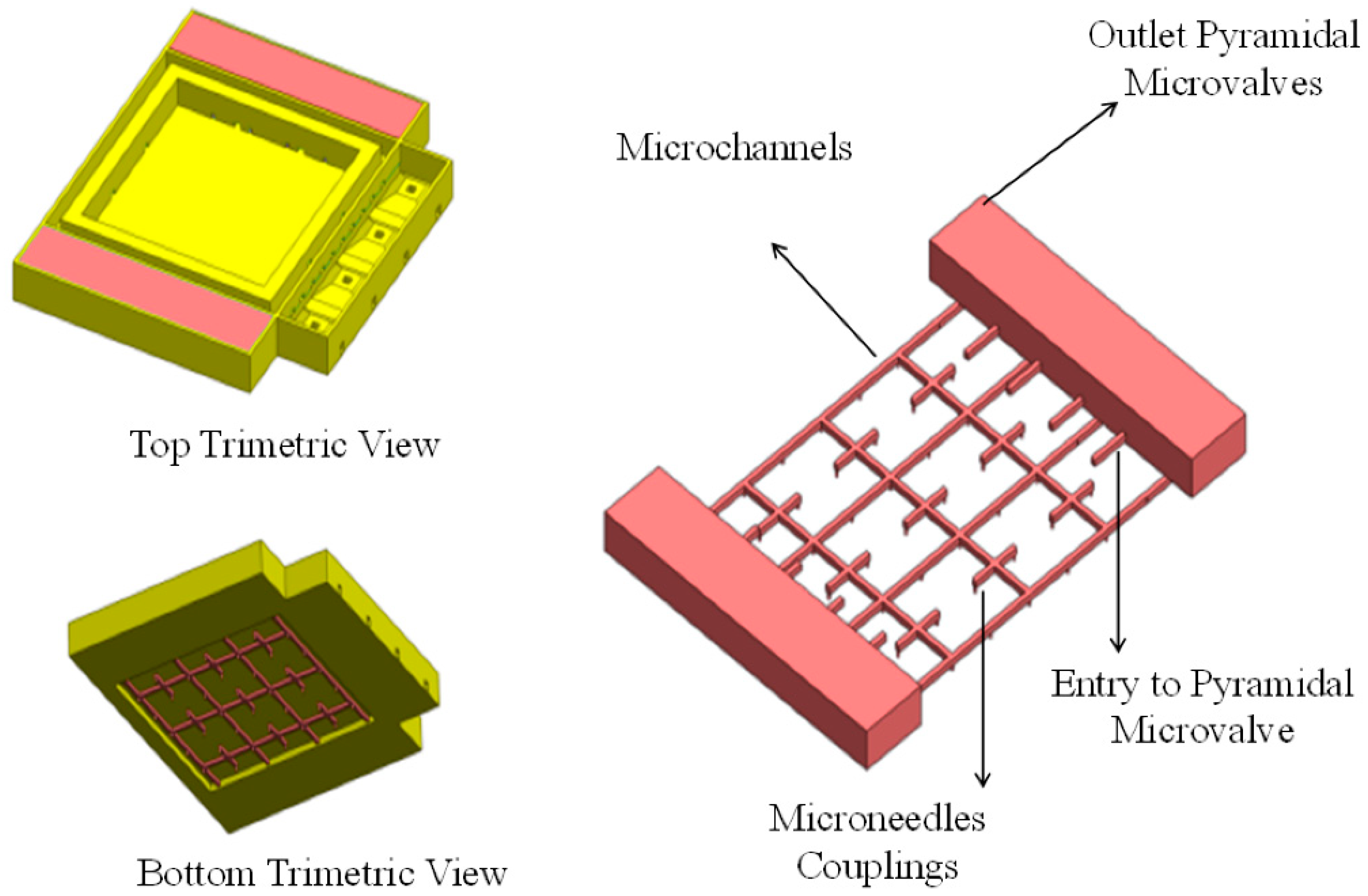
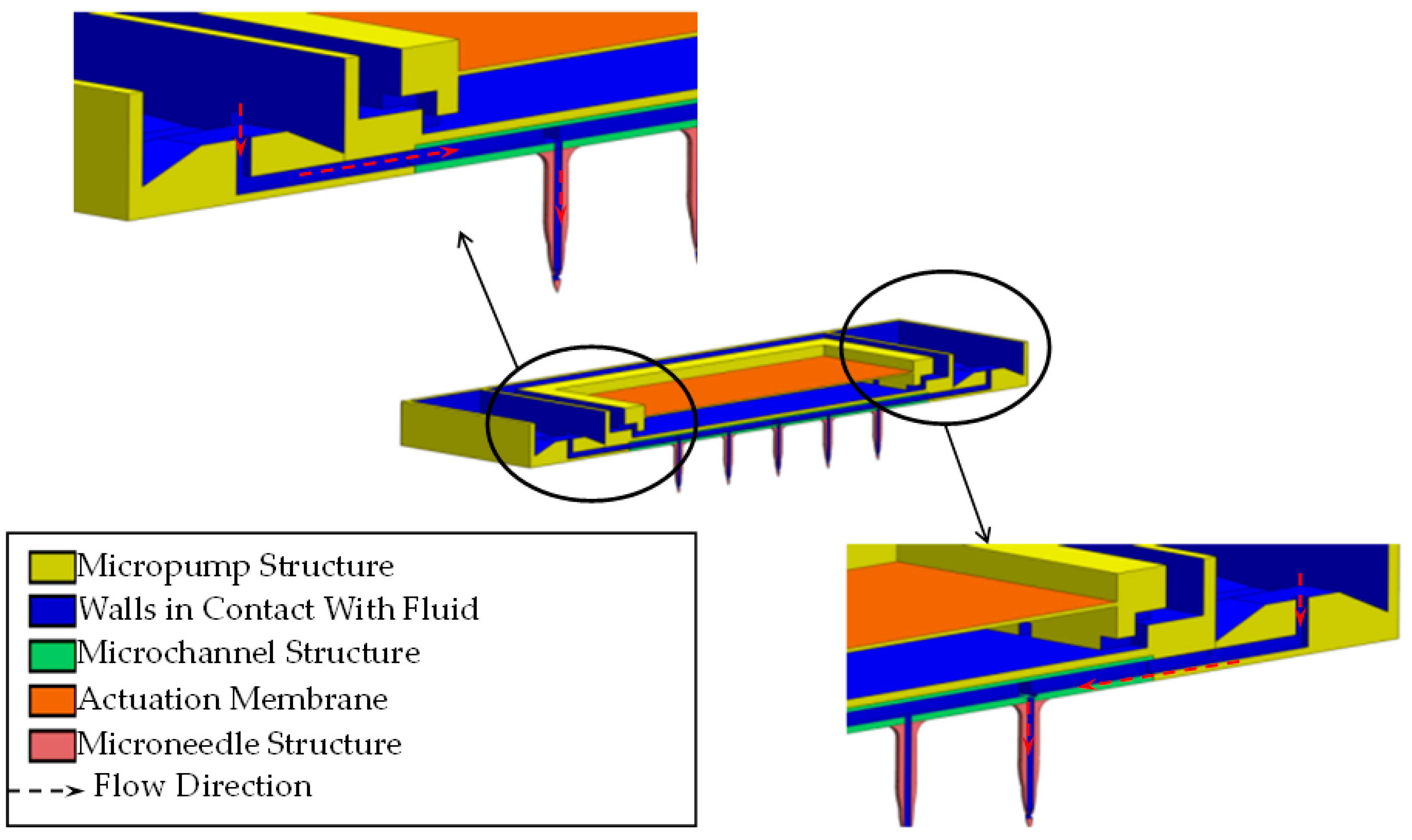
2.2. TD3 Structural Design
2.2.1. Suction Oriented Structural Design
2.2.2. Discharge Oriented Structural Design
2.3. TD3 Theoretical Calculations
3. TD3 Computational Fluid Dynamics (CFD) Approach
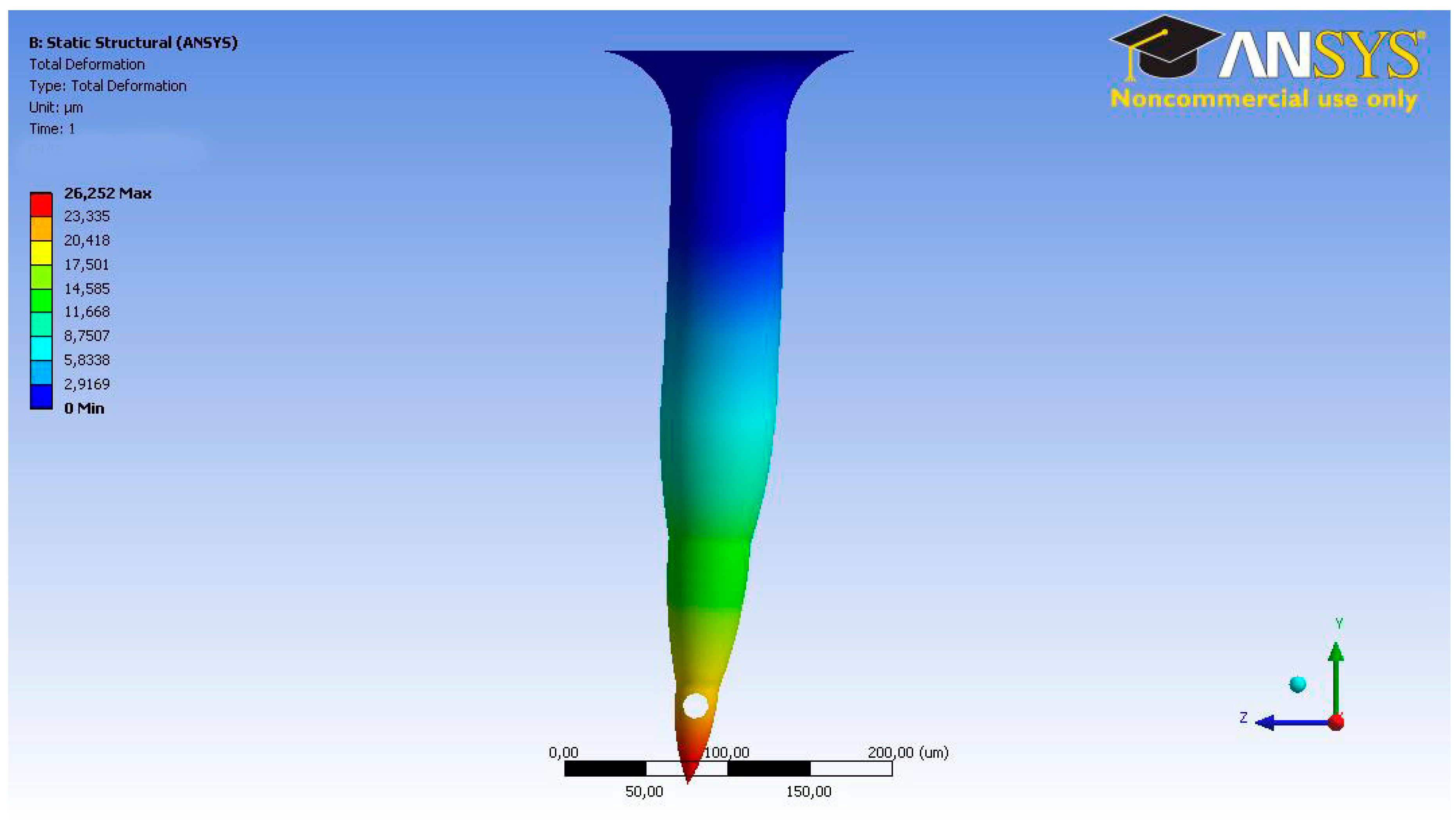
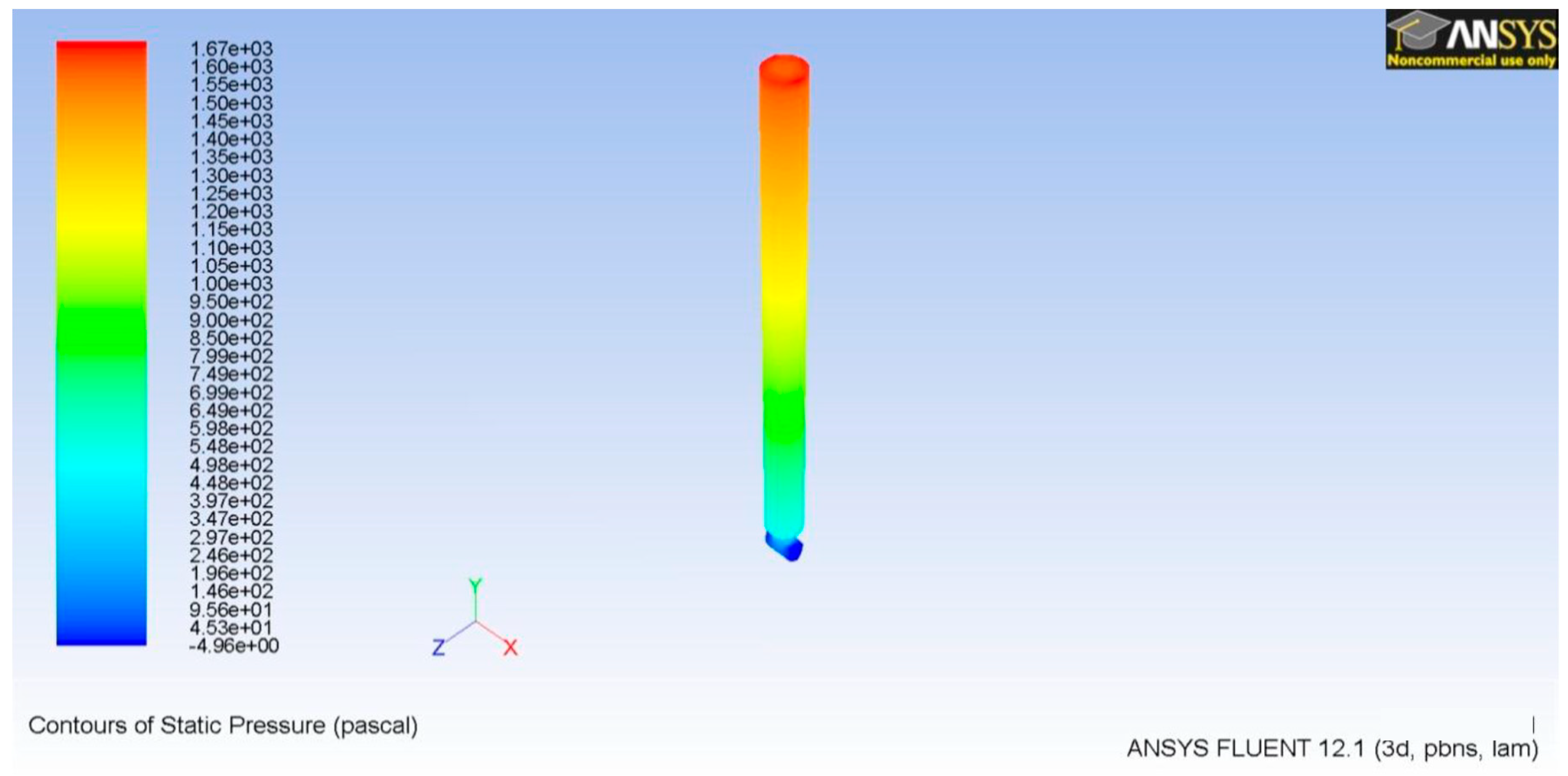
3.1. Microneedle CFD
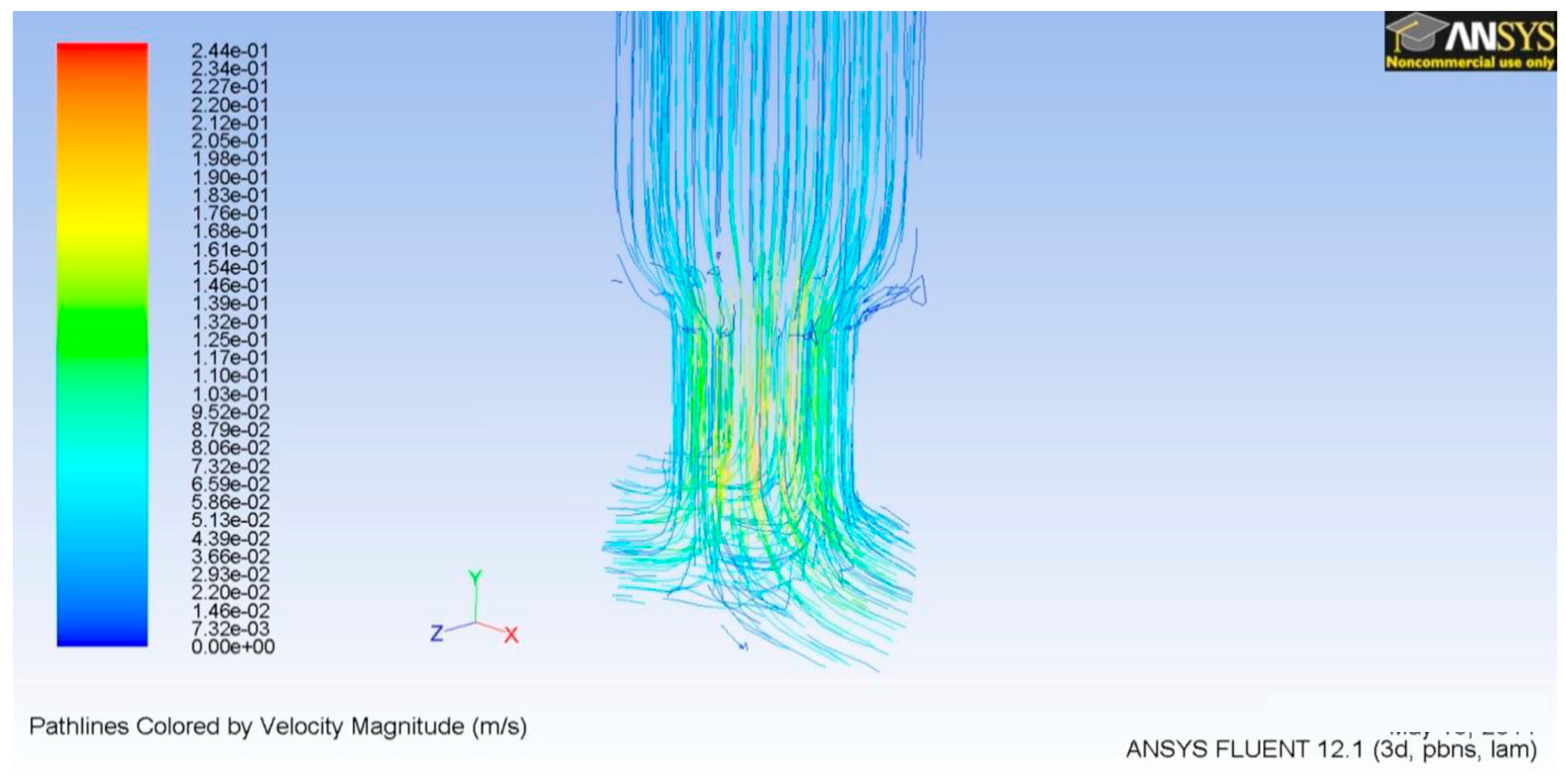
3.2. TD3 Discharge CFD
3.3. TD3 Suction CFD
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hood, R.R.; Kendall, E.L.; DeVoe, D.L.; Quezado, Z.; Junqueira, M.J.; Finkel, C.; Vreeland, W.N. Microfluidic formation of nanoscale liposomes for passive transdermal drug delivery. In Proceedings of the Microsystems for Measurement and Instrumentation (MAMNA), Gaithersburg, MD, USA, 14 May 2013; pp. 12–15. [Google Scholar]
- Dolżan, T.; Vrtačnik, D.; Resnik, D.; Aljančič, U.; Możek, M.; Pečar, B.; Amon, S. Design of transdermal drug delivery system with PZT actuated micropump. In Proceedings of the 37th International Convention on Information and Communication Technology, Electronics and Microelectronics (MIPRO), Opatija, Croatia, 26–30 May 2014; pp. 96–99. [Google Scholar]
- Lee, H.; Song, C.; Baik, S.; Kim, D.; Hyeon, T.; Kim, D.H. Device-assisted transdermal drug delivery. Adv. Drug Deliv. Rev. 2018, 127, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Mousoulis, C.; Ochoa, M.; Papageorgiou, D.; Ziaie, B. A Skin-Contact-Actuated Micropump for Transdermal Drug Delivery. IEEE Trans. Biomed. Eng. 2011, 58, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Camović, M.; Biščević, A.; Brčić, I.; Borčak, K.; Bušatlić, S.; Ćenanović, N.; Mulalić, A.; Osmanlić, M.; Sirbubalo, M.; Tucak, A.; et al. Coated 3d printed PLA microneedles as transdermal drug delivery systems. IFMBE Proc. 2020, 73, 735–742. [Google Scholar] [CrossRef]
- Wang, W.; Soper, S.A. Bio-MEMS Technologies and Applications, 1st ed.; CRC Press: Boca Raton, NY, USA, 2006; pp. 7–237. ISBN 9780849335327. [Google Scholar]
- Ashraf, M.W.; Tayyaba, S.; Afzulpurkr, N. Tapered tip hollow silicon microneedles for transdermal drug delivery. In Proceedings of the 2nd International Conference on Mechanical and Electronics Engineering (ICMEE), Kyoto, Japan, 1–3 August 2010. [Google Scholar]
- Jurcicek, P.; Zou, H.; Zhang, S.; Liu, C. Design and fabrication of hollow out-of-plane silicon microneedles. IET Micro Nano Lett. 2013, 8, 78–81. [Google Scholar] [CrossRef]
- Varadan, V.K.; Vinoy, K.J.; Gopalakrishnan, S. Smart Material Systems and MEMS: Design and Development Methodologies, 1st ed.; John Wiley & Sons: Chichester, UK, 2006; ISBN 9780470093610. [Google Scholar]
- Cong, W.; Jin-seong, K.; Jungyul, P. Micro check valve integrated magnetically actuated micropump for implantable drug delivery. In Proceedings of the 19th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Kaohsiung, Taiwan, 18–22 June 2017; pp. 1711–1713. [Google Scholar]
- Shoji, E. Fabrication of a diaphragm micropump system utilizing the ionomer-based polymer actuator. Sens. Actuators B Chem. 2016, 237, 660–665. [Google Scholar] [CrossRef]
- Garcia, J.; Rios, I.; Fonthal, F. Structural and microfluidic analysis of microneedle array for drug delivery. In Proceedings of the 31st Symposium on Microelectronics Technology and Devices IEEE SBMicro 2016, Belo Horizonte, Brazil, 29 August–3 September 2016; pp. 1–4. [Google Scholar]
- Kawun, P.; Leahy, S.; Lai, Y. A thin PDMS nozzle/diffuser micropump for biomedical applications. Sens. Actuators B Chem. 2016, 249, 149–154. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, N.; George, D.; Sen, A.K. Analytical modeling, simulations and experimental studies of a PZT actuated planar valveless PDMS micropump. Sens. Actuators B Chem. 2015, 225, 81–94. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Mousavi, S.A.; Kashaninejad, N.; Phan, D.T. Design, fabrication and characterization of drug delivery systems based on lab-on-a-chip technology. Adv. Drug Deliv. Rev. 2013, 65, 1403–1419. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.P.; Martanto, W.; Allen, M.G.; Prausnitz, M.R. Hollow metal microneedles for insulin delivery to diabetic rats. IEEE Trans. Biomed. Eng. 2005, 52, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Roxhed, N.T.; Gasser, C.; Griss, P.; Holzapfel, G.A.; Stemme, G. Penetration-enhanced ultrasharp microneedles and prediction on skin interaction for efficient transdermal drug delivery. J. Microelectromech. Syst. 2007, 16, 1429–1440. [Google Scholar] [CrossRef]
- Sanjay, S.T.; Zhou, W.; Dou, M.; Tavakoli, H.; Ma, L.; Xu, F.; Li, X. Recent advances of controlled drug delivery using microfluidic platforms. Adv. Drug Deliv. Rev. 2018, 128, 3–28. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.J.; Xie, D.L.; Zhang, J.P.; Chang, W.R.; Liang, D.C. Crystal structure of desheptapeptide (B24–B30) insulin at 1.6 Å resolution: Implications for receptor binding. Proc. Natl. Acad. Sci. USA 1997, 94, 2975–2980. [Google Scholar] [CrossRef] [PubMed] [Green Version]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, J.; Ríos, I.; Fonthal Rico, F.
Design and Analyses of a Transdermal Drug Delivery Device (TD3)
García J, Ríos I, Fonthal Rico F.
Design and Analyses of a Transdermal Drug Delivery Device (TD3)
García, Jennifer, Ismael Ríos, and Faruk Fonthal Rico.
2019. "Design and Analyses of a Transdermal Drug Delivery Device (TD3)
García, J., Ríos, I., & Fonthal Rico, F.
(2019). Design and Analyses of a Transdermal Drug Delivery Device (TD3)





