Detection of Triacetone Triperoxide (TATP) Precursors with an Array of Sensors Based on MoS2/RGO Composites
Abstract
:1. Introduction
2. Materials and Methods
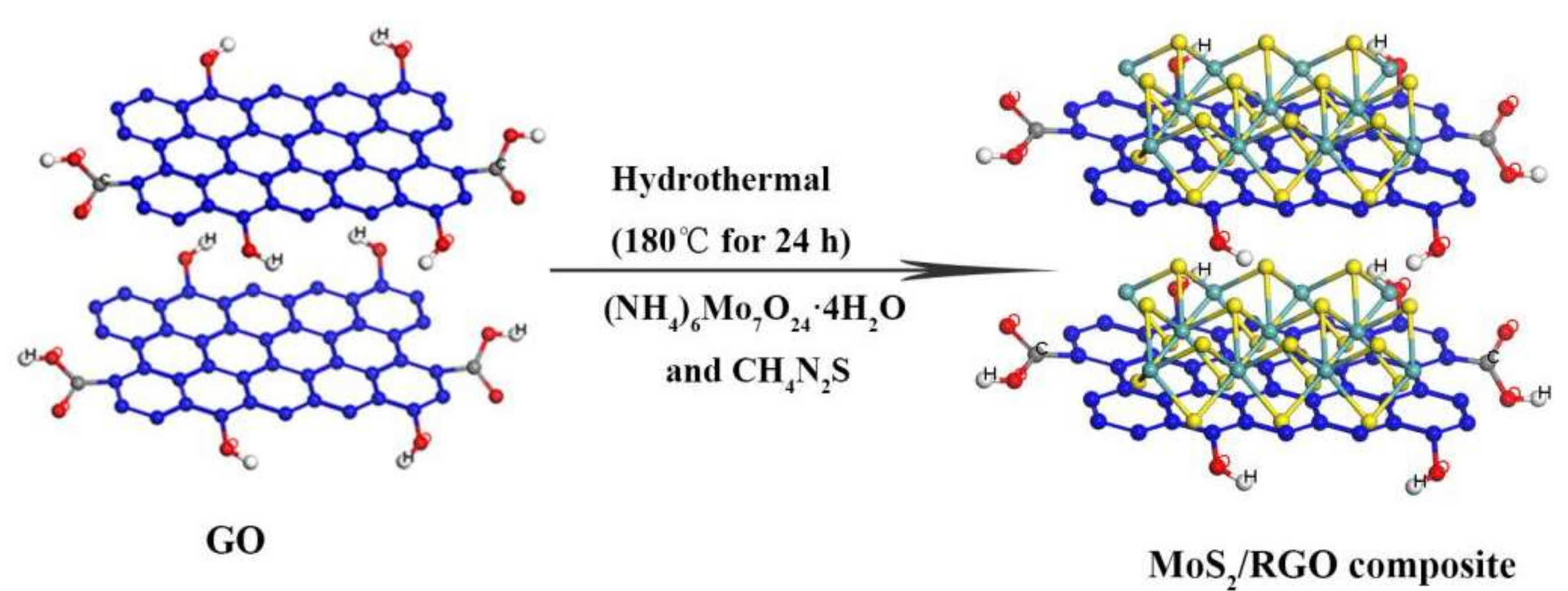
2.1. Preparation of MoS2/RGO Composites
2.2. Characterization
2.3. Manufacture and Testing of Sensor Parts
3. Results and Discussion
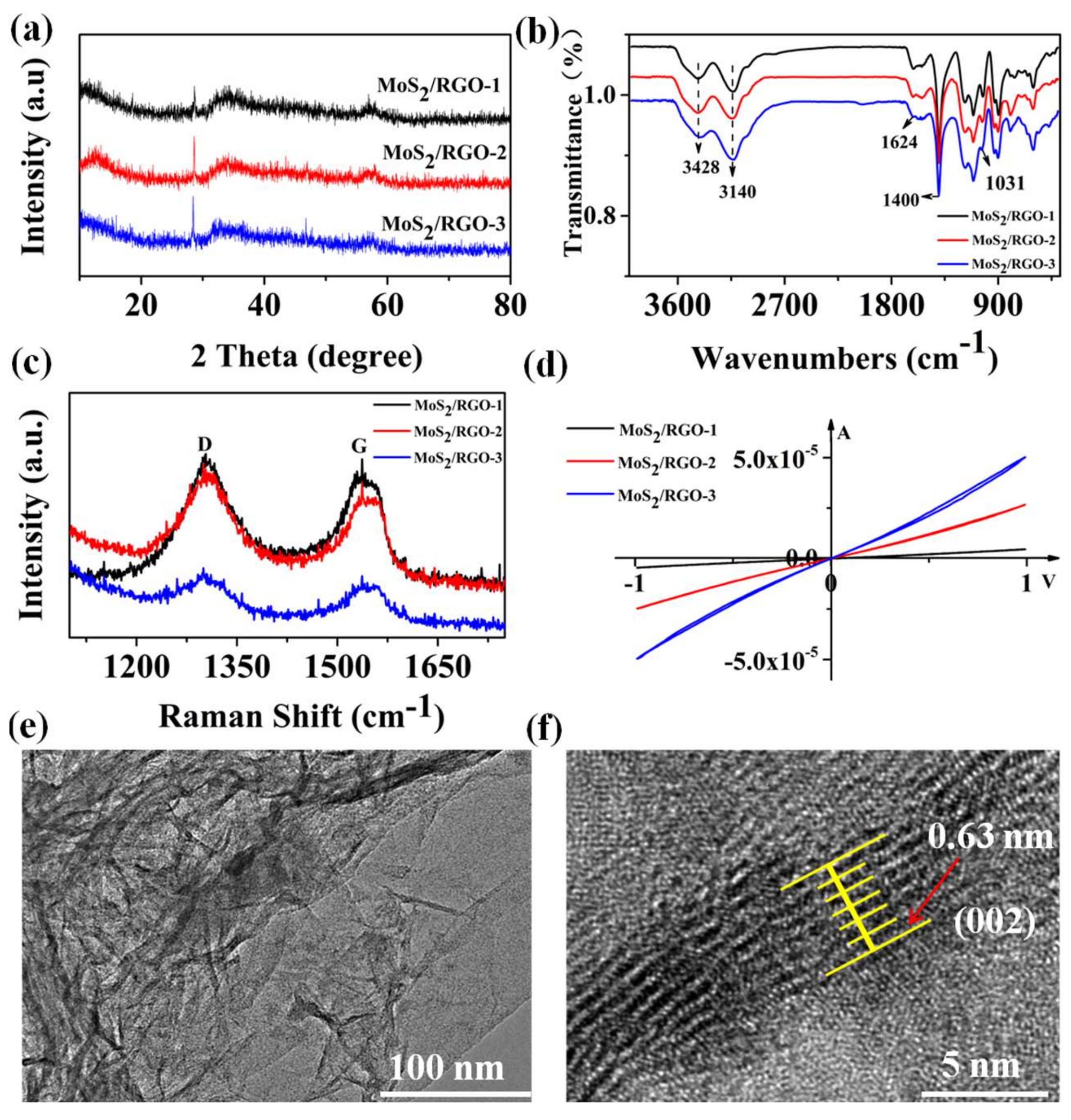
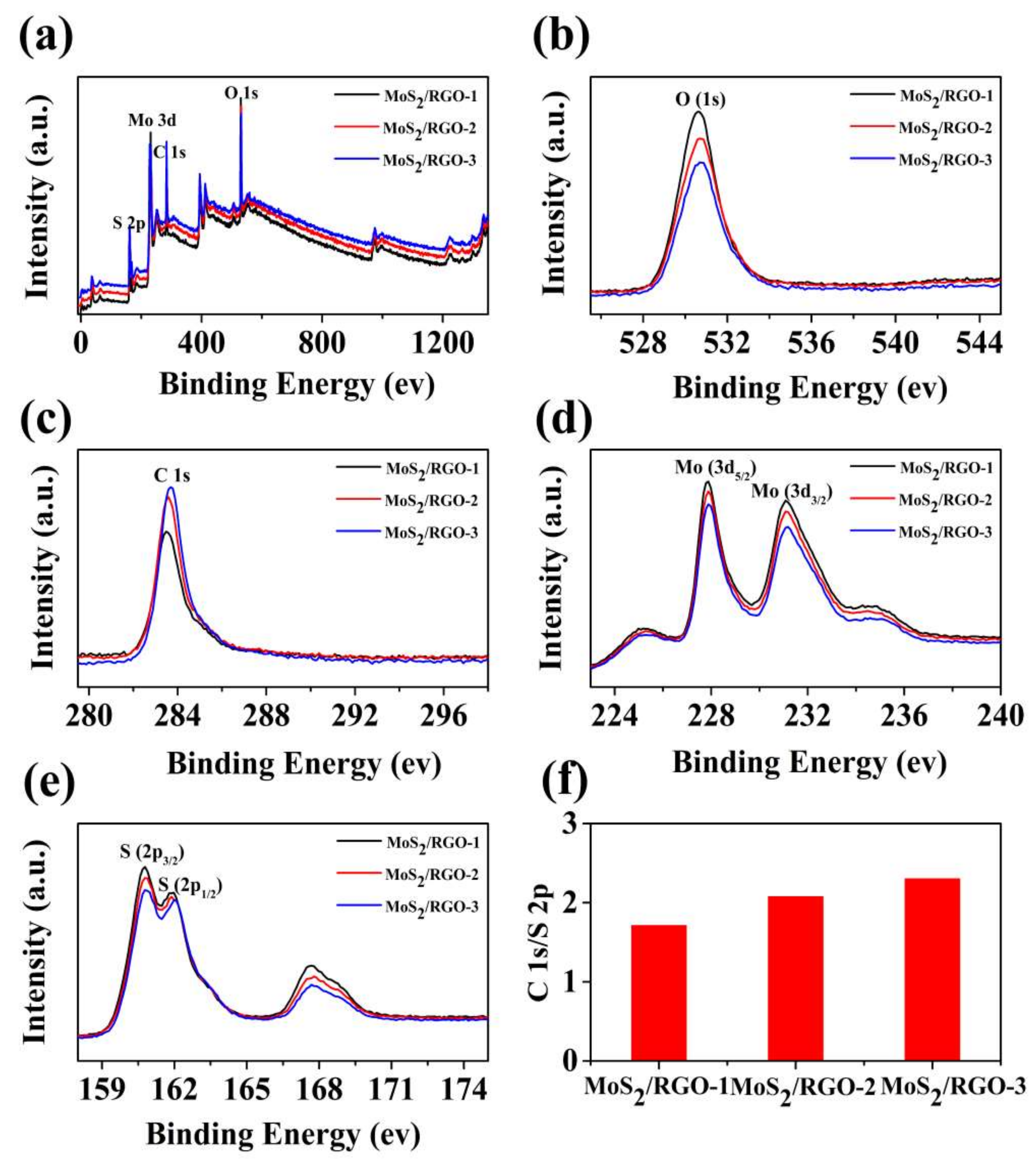
3.1. Characterization Results of MoS2/RGO
3.2. Fabrication and Testing of Sensor Array
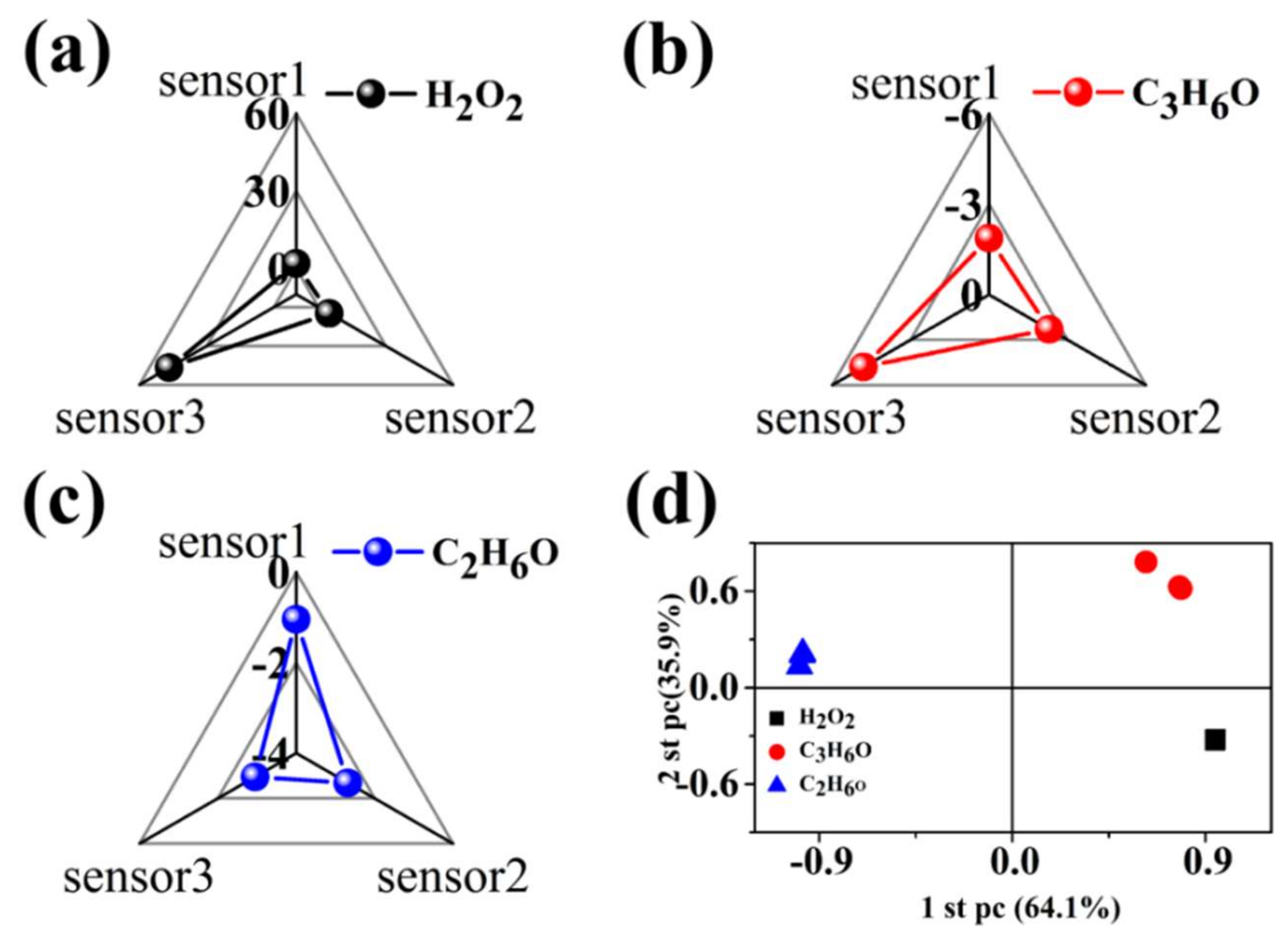
3.3. Discriminative Capability of the Sensor Array
3.4. Analysis of the Possible Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Suman, P.; Wujian, M. Sensitive determination of triacetone triperoxide explosives using electrogenerated chemiluminescence. Anal. Chem. 2013, 85, 8008–8015. [Google Scholar]
- Beveridge, A. Forensic Investigation of Explosions; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Capua, E.; Cao, R.; Sukenik, C.N.; Naaman, R. Detection of triacetone triperoxide (TATP) with an array of sensors based on non-specific interactions. Sens. Actuators B Chem. 2009, 140, 122–127. [Google Scholar] [CrossRef]
- Dubnikova, F.; Kosloff, R.; Zeiri, Y.; Karpas, Z. Novel Approach to the Detection of Triacetone Triperoxide (TATP): Its Structure and Its Complexes with Ions. Phys. Chem. A 2002, 106, 4951–4956. [Google Scholar] [CrossRef]
- Muller, D.; Levy, A.; Shelef, R.; Abramovich-Bar, S.; Sonenfeld, D.; Tamiri, T. Improved method for the detection of TATP after explosion. J. Forensic Sci. 2004, 49, 935–938. [Google Scholar] [CrossRef]
- Oxley, J.C.; Smith, J.L.; Shinde, K.; Moran, J. Determination of the Vapor Density of Triacetone Triperoxide (TATP) Using a Gas Chromatography Headspace Technique. Propellants Explos. Pyrotech. 2010, 30, 127–130. [Google Scholar] [CrossRef]
- Mamo, S.K.; Gonzalez-Rodriguez, J. Development of a molecularly imprinted polymer-based sensor for the electrochemical determination of triacetone triperoxide (TATP). Sensors 2014, 14, 23269–23282. [Google Scholar] [CrossRef]
- Hengwei, L.; Suslick, K.S. A colorimetric sensor array for detection of triacetone triperoxide vapor. J. Am. Chem. Soc. 2010, 132, 15519–15521. [Google Scholar]
- Fan, W.; Young, M.; Canino, J.; Smith, J.; Oxley, J.; Almirall, J.R. Fast detection of triacetone triperoxide (TATP) from headspace using planar solid-phase microextraction (PSPME) coupled to an IMS detector. Anal. Bioanal. Chem. 2012, 403, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Peng, L.; Wen, M.; Zhou, Q.; Chen, C.; Wang, X.; Chen, W.; Li, H. Dopant-Assisted Positive Photoionization Ion Mobility Spectrometry Coupled with Time-Resolved Thermal Desorption for on-site Detection of TATP and HMTD in Complex Matrices. Anal. Chem. 2016, 88, 4391–4399. [Google Scholar] [CrossRef]
- Lubczyk, D.; Siering, C.; Lörgen, J.; Shifrina, Z.B.; Müllen, K.; Waldvogel, S.R. Simple and sensitive online detection of triacetone triperoxide explosive. Sens. Actuators B Chem. 2010, 143, 561–566. [Google Scholar] [CrossRef]
- Burks, R.M.; Hage, D.S. Current trends in the detection of peroxide-based explosives. Anal. Bioanal. Chem. 2009, 395, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Buttigieg, G.A.; Knight, A.K.; Denson, S.; Pommier, C.; Denton, M.B. Characterization of the explosive tricetone triperoxide and detection by ion mobility spectrometry. Forensic Sci. Int. 2003, 135, 53–59. [Google Scholar] [CrossRef]
- Salb, C.; Wackerbarth, H.; Christou, K.; Gundrum, L.; Niederkrüger, M.; Beushausen, V.; Viöl, W. Detection of explosives based on surface-enhanced Raman spectroscopy. Appl. Opt. 2010, 49, 4362–4366. [Google Scholar]
- Leo, W.; Stuart, W.; Konrad, S.; Andrew, C. Development of an LC/MS method for the trace analysis of triacetone triperoxide (TATP). Analyst 2002, 127, 1627–1632. [Google Scholar]
- Zitrin, S. Decomposition products of petn in post explosion analysis. J. Energ. Mater. 1986, 4, 77–91. [Google Scholar]
- Fang, X.; Ahmad, S.R. Detection of explosive vapour using surface-enhanced Raman spectroscopy. Appl. Phys. B 2009, 97, 723–726. [Google Scholar] [CrossRef]
- Cotte-Rodríguez, I.; Chen, H.; Cooks, R.G. Rapid trace detection of triacetone triperoxide (TATP) by complexation reactions during desorption electrospray ionization. Chem. Commun. 2006, 9, 953–955. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Tao, L. A novel array of chemiluminescence sensors for sensitive, rapid and high-throughput detection of explosive triacetone triperoxide at the scene. Biosens. Bioelectron. 2013, 47, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Song, M.J.; Hwang, S.W.; Whang, D. Non-enzymatic electrochemical CuO nanoflowers sensor for hydrogen peroxide detection. Talanta 2010, 80, 1648–1652. [Google Scholar] [CrossRef]
- Luo, L.; Li, F.; Zhu, L.; Zhang, Z.; Ding, Y.; Deng, D. Non-enzymatic hydrogen peroxide sensor based on MnO2-ordered mesoporous carbon composite modified electrode. Electrochim. Acta 2012, 77, 179–183. [Google Scholar] [CrossRef]
- Shen, J.-Y.; Wang, M.-D.; Wang, Y.-F.; Hu, J.-Y.; Zhu, Y.; Zhang, Y.X.; Li, Z.-J.; Yao, H.-C. Iron and carbon codoped WO3 with hierarchical walnut-like microstructure for highly sensitive and selective acetone sensor. Sens. Actuators B Chem. 2018, 256, 27–37. [Google Scholar] [CrossRef]
- Osica, I.; Imamura, G.; Shiba, K.; Ji, Q.; Shrestha, L.K.; Hill, J.P.; Kurzydlowski, K.J.; Yoshikawa, G.; Ariga, K. Highly Networked Capsular Silica-Porphyrin Hybrid Nanostructures as Efficient Materials for Acetone Vapor Sensing. ACS Appl. Mater. Interfaces 2017, 9, 9945–9954. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Koo, W.T.; Jang, J.S.; Kim, D.H.; Kim, M.H.; Kim, I.D. Nanoscale PtO2 Catalysts-Loaded SnO2 Multichannel Nanofibers toward Highly Sensitive Acetone Sensor. ACS Appl. Mater. Interfaces 2018, 10, 2016–2025. [Google Scholar] [CrossRef]
- Donglai, L.; Avi, C.; Munoz, R.A.A.; Tanin, T.; Joseph, W. Highly sensitive electrochemical detection of trace liquid peroxide explosives at a Prussian-blue ‘artificial-peroxidase’ modified electrode. Analyst 2006, 131, 1279–1281. [Google Scholar]
- Xie, Y.; Cheng, I.F. Selective and rapid detection of triacetone triperoxide by double-step chronoamperometry. Microchem. J. 2010, 94, 166–170. [Google Scholar] [CrossRef]
- Dobrokhotov, V.; Oakes, L.; Sowell, D.; Larin, A.; Hall, J.; Kengne, A.; Bakharev, P.; Corti, G.; Cantrell, T.; Prakash, T. Toward the nanospring-based artificial olfactory system for trace-detection of flammable and explosive vapors. Sens. Actuators B Chem. 2012, 168, 138–148. [Google Scholar] [CrossRef]
- Zhang, W.H.; Zhang, W.D.; Chen, L.Y. Highly sensitive detection of explosive triacetone triperoxide by an In2O3 sensor. Nanotechnology 2010, 21, 315502. [Google Scholar] [CrossRef]
- Warmer, J.; Wagner, P.; Schöning, M.J.; Kaul, P. Detection of triacetone triperoxide using temperature cycled metal-oxide semiconductor gas sensors: Detection of triacetone triperoxide. Phys. Status Solidi A Appl. Mater. Sci. 2015, 212, 1289–1298. [Google Scholar] [CrossRef]
- Fowler, J.D.; Allen, M.J.; Tung, V.C.; Yang, Y.; Kaner, R.B.; Weiller, B.H. Practical Chemical Sensors from Chemically Derived Graphene. ACS Nano 2009, 3, 301–306. [Google Scholar] [CrossRef] [Green Version]
- Yuan, W.; Shi, G. Graphene-based gas sensors. J. Mater. Chem. A 2013, 1, 10078–10091. [Google Scholar] [CrossRef]
- Huang, B.; Li, Z.; Liu, Z.; Zhou, G.; Hao, S.; Wu, J.; Gu, B.L.; Duan, W. Adsorption of Gas Molecules on Graphene Nanoribbons and Its Implication for Nanoscale Molecule Sensor. J. Phys. Chem. C 2008, 112, 13442–13446. [Google Scholar] [CrossRef] [Green Version]
- Mao, S.; Cui, S.; Lu, G.; Yu, K.; Wen, Z.; Chen, J. Tuning gas-sensing properties of reduced graphene oxide using tin oxide nanocrystals. J. Mater. Chem. 2012, 22, 11009–11013. [Google Scholar] [CrossRef]
- Vedala, H.; Sorescu, D.C.; Kotchey, G.P.; Star, A. Chemical sensitivity of graphene edges decorated with metal nanoparticles. Nano Lett. 2011, 11, 2342–2347. [Google Scholar] [CrossRef]
- Jung, M.W.; Kang, S.M.; Nam, K.-H.; An, K.-S.; Ku, B.-C. Highly transparent and flexible NO2 gas sensor film based on MoS2/rGO composites using soft lithographic patterning. Appl. Surf. Sci. 2018, 456, 7–12. [Google Scholar] [CrossRef]
- Xian, L.; Jing, W.; Dan, X.; Xu, J.; Yi, X.; Lan, X.; Komarneni, S. Reduced graphene oxide/MoS2 hybrid films for room-temperature formaldehyde detection. Mater. Lett. 2016, 189, 42–45. [Google Scholar]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef]
- Ganatra, R.; Zhang, Q. Few-Layer MoS2: A Promising Layered Semiconductor. ACS Nano 2014, 8, 4074–4099. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, Y.; Murali, S.; Stoller, M.D.; Ruoff, R.S. Nanostructured Reduced Graphene Oxide/Fe2O3 Composite as a High-Performance Anode Material for Lithium Ion Batteries. ACS Nano 2011, 5, 3333–3338. [Google Scholar] [CrossRef]
- Zu, S.Z.; Han, B.H. Aqueous Dispersion of Graphene Sheets Stabilized by Pluronic Copolymers: Formation of Supramolecular Hydrogel. J. Phys. Chem. C 2009, 113, 13651–13657. [Google Scholar] [CrossRef]
- Shao, L.; Wu, Z.; Duan, H.; Shaymurat, T. Discriminative and rapid detection of ozone realized by sensor array of Zn2+ doping tailored MoS2 ultrathin nanosheets. Sens. Actuators B Chem. 2018, 258, 937–946. [Google Scholar] [CrossRef]
- Chen, X.; Meng, D.; Wang, B.; Li, B.W.; Li, W.; Bielawski, C.W.; Ruoff, R.S. Rapid thermal decomposition of confined graphene oxide films in air. Carbon 2016, 101, 71–76. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, H.; Yan, C.; Yang, Z.; Zhu, G.; Gao, J.; Yin, F.; Wang, C. Fabrication of conductive graphene oxide-WO3 composite nanofibers by electrospinning and their enhanced acetone gas sensing properties. Sens. Actuators B Chem. 2018, 264, 128–138. [Google Scholar] [CrossRef]
- Li, Q.; Guo, B.; Yu, J.; Ran, J.; Zhang, B.; Yan, H.; Gong, J.R. Highly efficient visible-light-driven photocatalytic hydrogen production of CdS-cluster-decorated graphene nanosheets. Am. Chem. Soc. 2011, 133, 10878–10884. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, R.; Jiao, W.; Ding, G.; Hao, L.; Yang, F.; He, X. MoS2 graphene fiber based gas sensing devices. Carbon 2015, 95, 34–41. [Google Scholar] [CrossRef]
- Ghasemi, F.; Jalali, M.; Abdollahi, A.; Mohammadi, S.; Sanaee, Z.; Mohajerzadeh, S. A high performance supercapacitor based on decoration of MoS2/reduced graphene oxide with NiO nanoparticles. RSC Adv. 2017, 7, 52772–52781. [Google Scholar] [CrossRef]
- Lipatov, A.; Varezhnikov, A.; Wilson, P.; Sysoev, V.; Kolmakov, A.; Sinitskii, A. Highly selective gas sensor arrays based on thermally reduced graphene oxide. Nanoscale 2013, 5, 5426–5434. [Google Scholar] [CrossRef]
- Kumar, R.; Goel, N.; Kumar, M. High performance NO2 sensor using MoS2 nanowires network. Appl. Phys. Lett. 2018, 112, 053502. [Google Scholar] [CrossRef]
- Saraf, M.; Natarajan, K.; Saini, A.K.; Mobin, S.M. Small biomolecule sensors based on an innovative MoS2-rGO heterostructure modified electrode platform: A binder-free approach. Dalton Trans. 2017, 46, 15848–15858. [Google Scholar] [CrossRef]
- Li, H.; Yu, K.; Li, C.; Tang, Z.; Guo, B.; Lei, X.; Fu, H.; Zhu, Z. Charge-Transfer Induced High Efficient Hydrogen Evolution of MoS2/graphene Cocatalyst. Sci. Rep. 2015, 5, 18730. [Google Scholar] [CrossRef]
- Lu, Y.; Meyyappan, M.; Li, J. Trace detection of hydrogen peroxide vapor using a carbon-nanotube-based chemical sensor. Small 2011, 7, 1714–1718. [Google Scholar] [CrossRef]
- Lee, D.J.; Choi, S.W.; Byun, Y.T. Room temperature monitoring of hydrogen peroxide vapor using platinum nanoparticles-decorated single-walled carbon nanotube networks. Sens. Actuators B Chem. 2017, 256, 744–750. [Google Scholar] [CrossRef]
- Verma, A.L.; Saxena, S.; Saini, G.S.S.; Gaur, V.; Jain, V.K. Hydrogen peroxide vapor sensor using metal-phthalocyanine functionalized carbon nanotubes. Thin Solid Films 2011, 519, 8144–8148. [Google Scholar] [CrossRef]
- Lichtenstein, A.; Havivi, E.; Shacham, R.; Hahamy, E.; Leibovich, R.; Pevzner, A.; Krivitsky, V.; Davivi, G.; Presman, I.; Elnathan, R. Supersensitive fingerprinting of explosives by chemically modified nanosensors arrays. Nat. Commun. 2014, 5, 4195. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Zhou, C.; Zu, B.; Li, Y.; Dou, X. Contactless and Rapid Discrimination of Improvised Explosives Realized by Mn2+ Doping Tailored ZnS Nanocrystals. Adv. Funct. Mater. 2016, 26, 4578–4586. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Principal component analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef] [Green Version]
- Jolliffe, I.T. Principal component analysis. J. Mark. Res. 2002, 87, 513. [Google Scholar]
- Lu, Y.; Partridge, C.; Meyyappan, M.; Jing, L. A carbon nanotube sensor array for sensitive gas discrimination using principal component analysis. J. Electroanal. Chem. 2006, 593, 105–110. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Zhang, J.; Liang, X.; Duan, H. Density functional theory study of the adsorption of hydrogen atoms on Cu2X (X = 3d) clusters. Chem. Phys. Lett. 2016, 651, 137–143. [Google Scholar] [CrossRef]
- Bhati, V.S.; Ranwa, S.; Rajamani, S.; Kumari, K.; Raliya, R.; Biswas, P.; Kumar, M. Improved sensitivity with low limit of detection of hydrogen gas sensor based on rGO loaded Ni doped ZnO nanostructures. ACS Appl. Mater. Interfaces 2018, 10, 11116–11124. [Google Scholar] [CrossRef]
- Näther, N.; Henkel, H.; Schneider, A.; Schöning, M.J. Investigation of different catalytically active and passive materials for realising a hydrogen peroxide gas sensor. Phys. Status Solidi 2010, 206, 449–454. [Google Scholar] [CrossRef]



| Sensing Materials | Analyte Concentration | Temperature (°C) | Response (%) | Response Time | Ref. |
|---|---|---|---|---|---|
| SWCNTs | 100 ppm H2O2 | 25 | ~1.97 | ~20 s | [51] |
| Pt-SWCNTs | 60.6 ppm H2O2 | 23 ± 1 | ~50 | ~240 s | [52] |
| CuPc-f-MWNTs CoPc-f-MWNTs VPc-f-MWNTs | 34% H2O2 (aq) vapors | Ambient temperature | (−) 24.2 (+) 3.8 (−) 4.4 | 2 s 2 s 4 s | [53] |
| MoS2/RGO-3 | 50 ppm H2O2 | 25 | ~373.1 | ~9 s | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Q.; Wu, Z.; Duan, H.; Jia, D. Detection of Triacetone Triperoxide (TATP) Precursors with an Array of Sensors Based on MoS2/RGO Composites. Sensors 2019, 19, 1281. https://doi.org/10.3390/s19061281
Sun Q, Wu Z, Duan H, Jia D. Detection of Triacetone Triperoxide (TATP) Precursors with an Array of Sensors Based on MoS2/RGO Composites. Sensors. 2019; 19(6):1281. https://doi.org/10.3390/s19061281
Chicago/Turabian StyleSun, Qihua, Zhaofeng Wu, Haiming Duan, and Dianzeng Jia. 2019. "Detection of Triacetone Triperoxide (TATP) Precursors with an Array of Sensors Based on MoS2/RGO Composites" Sensors 19, no. 6: 1281. https://doi.org/10.3390/s19061281
APA StyleSun, Q., Wu, Z., Duan, H., & Jia, D. (2019). Detection of Triacetone Triperoxide (TATP) Precursors with an Array of Sensors Based on MoS2/RGO Composites. Sensors, 19(6), 1281. https://doi.org/10.3390/s19061281





