Advanced Micro- and Nano-Gas Sensor Technology: A Review
Abstract
:1. Introduction
2. Electrochemical Sensors
2.1. MOS Gas Sensors
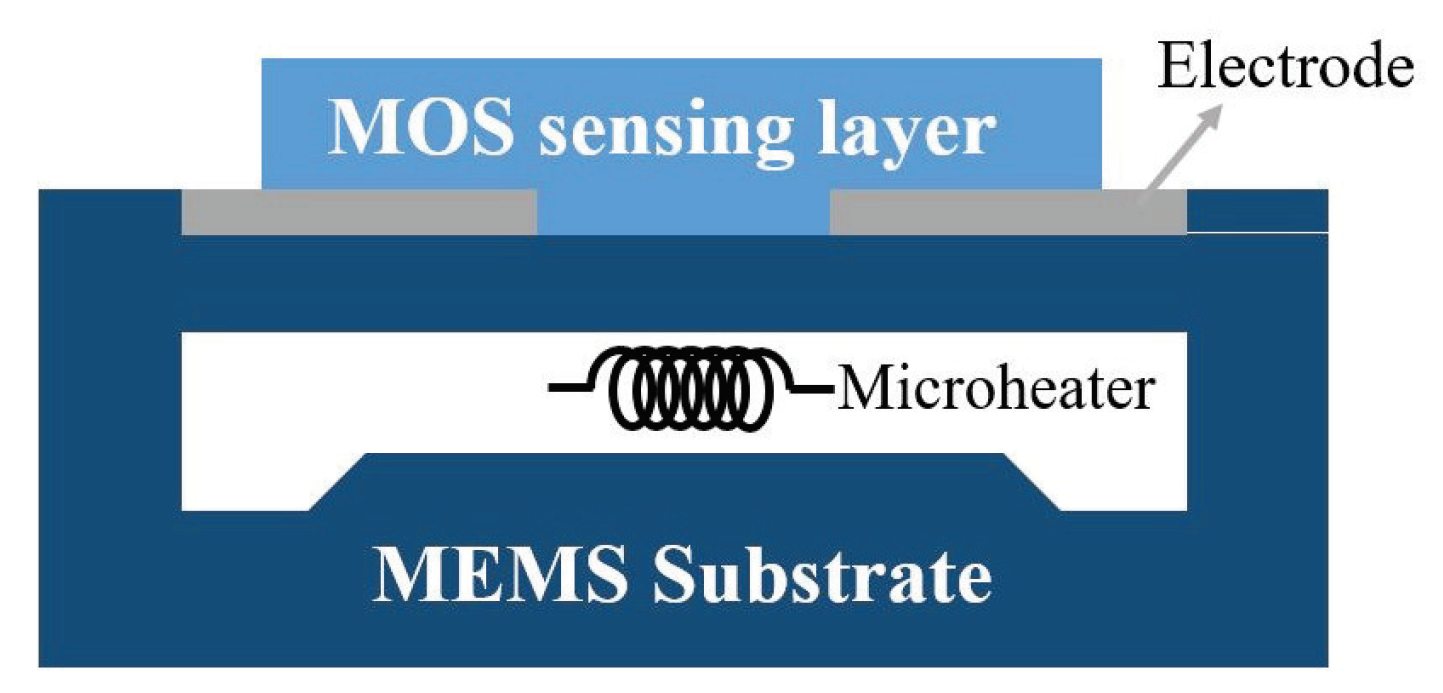
2.1.1. Structure and Mechanism
2.1.2. Fabrication
2.1.3. Applications
- MOS sensors for detection of VOCs: A MOS gas sensor in temperature cycled operation can be used to detect formaldehyde, benzene and naphthalene in ppb and sub-ppb concentrations with a varying background of ethanol with concentrations of up to 2 ppm [12]. It has been shown that selective detection of VOCs in the ppb range is possible even with an intensive background of various VOCs. However, sensitivity is reduced compared to the ideal laboratory case and in the absence of background VOCs. For ppb-level VOC detection, MOS gas sensors are designed with temperature cycled operation, which can achieve sufficient sensitivity and selectivity in combination with signal analysis based on pattern recognition. In the temperature cycled operation mode, the heater unit of the sensor is periodically set to different temperature steps and therefore, the MOS sensing layer goes through various states resulting in different level of interaction characteristics between the sensing layer and the target analyte at those specific temperature range [12,13]. Employing the temperature cycled MOS sensor in combination with the pattern recognition techniques can correlate the sensor response to the present analyte in a complex environment by providing an additional identifying parameter. Temperature cycled MOS sensors with detection capability of 100 ppb of formaldehyde and 20 ppb of naphthalene have been reported [12].
- MOS-based sensors with porous sensing layer: To enhance the interaction with the analytes and increase the reaction surface area, the porous structural MOS can be employed that offer high porosity, highly interconnected pore channels, and high surface area and active sites. These porous MOS sensors can be chemically synthesized via soft-templating method and nano-casting strategy [14], which enhances the device performance through facilitating the gas diffusion and improves the sensor’s sensitivity, response and recovery time, and selectivity. The tunability of this synthesis approach provides a potential to develop porous MOS sensors with various compositions, where pore size, film thickness, temperature, and humidity are the factors that affect sensing performance [15].
2.2. Organic-Based Chemiresistive Gas Sensors
2.2.1. Structure and Mechanism
2.2.2. Fabrication
2.2.3. Applications
- Capped nano-particle sensors for VOCs detection: Molecularly capped nano-particles have been intensively investigated as the chemiresistor sensing materials to detect various VOCs. Chemiresistor arrays have been fabricated with cross-linked Au nano-particle thin films with subtle structural differences. These chemiresistive sensors detect VOCs and breath biomarker under ambient conditions. The nano-particle composition and size are the key parameters in designing the sensor array with desired sensitivity, selectivity, and stability. This sensor configuration has been used for breath recognition of lung cancer patients. Nano-structured sensor arrays have shown high sensitivity and selectivity in detecting mixtures of VOCs with a LOD as low as 20 ppb [26].
- Polypyrrole sensors for detection of explosive gases: Chemiresistor gas sensor based on sulfonated dye-doped modified conducting polypyrrole (PPy) film has been designed and fabricated for highly sensitive detection of 2,4,6-trinitrotoluene that is one of the most commonly used explosives. The sensor uses electro synthesis of PPy on Au-IDEs in the presence of the sulfonated dyes and exhibits high sensitivity and good selectivity to TNT with no response to other related gases and with a LOD and linear dynamic range of about 0.2 ppb and 10–800 ppb, respectively [17].
3. CNT Gas Sensors
3.1. Structure and Mechanism
- Chemiresistive gas sorption CNT sensors: In these sensors exposing CNTs to the target gas results in charge transfer between the CNT and the gas. This phenomenon results in a change in conductivity of the CNT sensing material. The change in the device conductivity is correlated with the gas properties and concentration. In these sensors, recovery time can be improved by heating the sensing film [27]. The sensing property of sorption-based CNT gas sensors can be modified by using different chemical functional groups such as oxygen on the surface of CNTs where they can lead to selective interaction to desired analytes such as hydrogen-containing molecules. However, this can decrease the accessibility of the analytes to the CNT surface, hence reducing the sensitivity [29]. Common disadvantages of these sensors are long recovery time, irreversibility of CNT conductivity and decreased sensitivity for low gas energy levels [27].
- Gas ionization CNT sensors: High aspect ratio of CNTs provides an ideal geometry for creation of an electrical field by applying voltage. In ionization CNT gas sensors, CNTs are used for both anode and cathode electrodes to create electric field [30]. Analytes is ionized to be in plasma state by the accelerated electrons from the electrode. The ionization energy and the current though the plasma can be measured for identification of gas properties and concentration. This mechanism is useful for detection of gases with low sorption energy. Common gas ionization sensors are bulky with high energy consumption level; however, the use of CNTs can reduce the size and ionization energy of gas significantly due to the easier ionization enabled by CNT’s sharp tip structure and low work function [30,31].
- Capacitive-based and resonant frequency CNT sensors: CNTs can be used as the sensing element of capacitive-based gas sensors. In this structure, one plate of the capacitor is silicon and the other plate is made of is CNT coated silicon. By applying a voltage across the capacitor, CNT creates a high electric field which results in polarization of the gas molecules, and therefore, a change in the capacitance. The shift in the sensor’s capacitance is due to the dielectric constant change of CNT which is correlated with the target VOC concentration. This dielectric change of CNT sensor can also be used in a resonance frequency sensor configuration which measures the frequency shift associated with the gas properties and concentrations [32].
3.2. Fabrication
- Arc discharge technique: Since the synthesis temperature is above 1700 C, arc discharge technique has been reported to create less defects in the CNTs. In this method, fabrication is processed in helium, hydrogen, or methane-filled chamber containing graphite electrodes as shown in Figure 4. By applying the voltage, the electrode evaporates in gas and form CNTs on the other electrode [33,34].
- Pulsed laser ablation: PLA method has been used to produce SWCNTs of high quality and purity. The procedure is very similar to the arc discharge technique except that the energy is provided by a laser source. The laser is introduced onto the graphite layer which contains cobalt or other catalysts. A schematic diagram of PLA method is shown in Figure 5.
- Chemical vapor deposition: CVD has been proposed as an alternative to conventional CNT fabrication techniques due to its controllable process as well as high purity of its product.There are different CVD methods that have been used for CNT synthesis fabrication, such as catalyst CVD (CCVD), plasma enhanced CVD (PECVD) shown in Figure 6, radiofrequency CVD (RF-CVD), micro-wave plasma CVD (MPECVD) and water and oxygen assisted CVD. These days, CCVD and PECVD techniques are standard methods to synthesize CNTs, since they provide purer CNTs while using low temperature processes.
3.3. Application
- Gas sorption CNT sensors for NH, NO and organic compounds detection: Monolayer CNTs have been used for detection of NH and NO in mass adsorption principle. Limit of 44 ppb and 262 ppb have been reported for detection of NO and nitrotoluene, respectively [27]. Long recovery time up to 10 h is reported due to the very strong molecular bonds between some analytes and carbon, which is improved to 10 min by applying ultra-violet (UV) radiation to break bonds [27]. In addition, CNTs have been used in sensors employing field effect transistors (FET) to improve selectivity between NO, CO, CO, O and H [35,36].
- Gas sorption CNT sensors for CH and HO detection: CNT sensors have difficulty in detecting gases such as CO, water vapor and bimolecular gas since they are limited to detect molecules with high bonding energy and ability to transfer charges to the nano-tubes. To overcome this disadvantage, various doping of CNTs have been proposed. In mass adsorption gas sensors, CNTs doped with nitrogen and boron are reported to detect CH, HO and NO in a low concentration at room temperature. Boron doped CNTs have good sensitivity for ethylene (CH), while nitrogen doped CNTs are sensitive to NO and CO [37].
4. Acoustic Gas Sensors
4.1. QCM Gas Sensors
4.1.1. Structure and Mechanism
4.1.2. Fabrication
4.1.3. Applications
- Calixarene coated QCM for VOCs detection: QCM sensors with calixarene coating or calixarene derivatives have been employed for detection of analytes such as alcohols, halocarbons, esters, ethers, chemical warfare agents, and toxic gases. Calixarene modified QCM sensors exhibit strong sensing ability to methylene chloride emissions with a LOD of about 54 ppm. The cyclic structures, hydrogen-bonding capabilities, and highly organized properties of the calixarene derivatives play a key role in VOC detection. However, QCM sensing properties are affected due to the random arrangement of calixarene derivatives’ molecules on the surface of the crystal [44].
- PANI-ES coated QCM for vapor detection: QCM can be fabricated based on dip coated polyaniline emeraldine (PANI-ES) salt thin films. In these devices, three different acids thin films such as hydrochloric acid (HCl), dodecylbenzene sulfonic acid (DBSA) and 1,5-naphtalene disulfonic acids (1,5-NDSA) are doped on the AT-cut 10 MHz QCM electrode. These sensors exhibit a frequency shift linear to both the vapor concentrations in part per million (ppm) and the sensing film thickness in nano-meter (nm). The frequency changes in these sensors are mainly due to the electrostatic interactions between the dopant agents within PANI-ES films and the vapor molecules. PANI-DBSA films show a highly sensitive of ~7 Hz/ppm and selectivity to para-xylene over toluene and benzene with a LOD of 3 ppm. They exhibit a relatively short recovery time of less than 3 min and an acceptable sensitivity in the presence of humidity interference [44].
- Ultrasensitive PPy-BPB and -4BP-based QCM for gas detection: Polypyrrole-bromophenol blue (PPy-BPB) nano-structure-based QCM has been developed for the detection of very small trace amounts of nitro-explosive vapors. This ultrasensitive and selective QCM sensor uses PPy-BPB compound in nano-sphere and nano-rod forms on the gold electrode. At room temperature the sensors are stable, reversible, and exhibit fast response time. PPy modified QCM sensors can be doped with various bromine containing anion dopants to improve the sensor performance. The enhanced sensitivity of this sensor towards nitro-explosives is related to the non-covalent interaction of halogen-nitro synthons between the bromine atoms and nitro-explosive groups as electron deficient acceptors as well as the partial charge transfer interaction between the nitro-explosive groups and electron rich polymer film [45].
4.2. SAW Device
4.2.1. Structure and Mechanism
4.2.2. Fabrication
4.2.3. Applications
- Polymer-based SAW for biomarker and VOCs detection: SAW gas sensors can employ various polymers as their sensing element that can react to different analytes such as biomarkers associated with lung cancer. However, many of these polymers respond to the presence of more than one analyte. The number of chosen sensing materials and their properties are designed according to the type of biomarkers that need to be identified. Pattern recognition and neural network techniques are employed to discriminate various chemical analytes by analyzing signal obtained from these sensors with different sensing material [46].
- Palladium- and CuPc-based SAW for hydrogen detection: Palladium has been used as the sensing material on a SAW sensor to detect hydrogen. Absorbing and desorbing hydrogen molecules result in a change in density and electrical conductivity of the sensing material. Copper phthalocyanine (CuPc) has also been used in SAW system for hydrogen detection. It has been shown that CuPc layer alone is not sensitive enough to hydrogen, which required high operation temperature of more than 70 C. This operating temperature can be lowered by using CuPc or Pd thin film as sensing layer down to room temperature. In this design, the change in the sensor’s output is mainly due to the change in the electrical conductivity rather than the mass change of the sensing layer [49].
4.3. CMUTs
4.3.1. Structure and Mechanism
4.3.2. Fabrication
4.3.3. Application
- CMUT sensors for dimethyl methylphosphonate (DMMP) detection: Employing a very thin layer of DKAP polymer, CMUT sensors have been reported to detect DMMP, a simulant for sarin gas, with a good selectivity and a sensitivity of 48.8 zg/Hz/m [59]. In addition, polyisobutylene (PIB) coated CMUT sensor is also reported to detect DMMP with a sensitivity of 130 zg/Hz/m [51] with a minimum LOD for DMMP of 16.8 pptv [53].
- CMUT sensors for carbon dioxide detection: CMUT sensors employing different materials such as polyimide, amine-bearing functional groups and quinidine can be fabricated as a highly sensitive CO detector. A CO sensitivity of 1.06 ppm/Hz at 50 MHz and a resolution of 4.9 ppm in the ambient temperature has been reported with consideration of other influencing parameters such cross sensitivity with water vapor, sensor repeatability and regeneration [58].
5. Optical Gas Sensors
5.1. Fiber-Optic Gas Sensors
5.1.1. Structure and Mechanism
5.1.2. Fabrication
5.1.3. Applications
- Cholesteric liquid crystal film coated fiber-optic sensors for VOCs detection: A cholesteric liquid crystal film (CLCF) coated side polished fiber (SPF) has been used for VOC sensing where an increase in VOC concentration on CLCF results in an increase in the pitch of the resultant light. This creates a blue shift of the resonant dips which can be correlated with the exposed VOCs. The sensitivities of the CLCFC-SPF have been reported to be 7.08 nm.L/mmol, 3.46 nm.L/mmol and 0.52 nm.L/mmol for tetrahydrofuran, acetone, and methanol gas, respectively, where the sensitivity of CLCFC-SPF increases with the molar mass of the VOCs [64].
- ZnO nano-particle-based fiber-optic gas sensor: ZnO nano-particles coated fiber-optic sensors have shown concentration selectivity dependency for acetone, ammonia, and ethanol. The ZnO nano-particles show good sensitivity towards ammonia at low concentrations up to 150 ppm and acetone at high concentrations above 150 ppm due to the enhanced catalytic reactivity of acetone at high concentrations [65].
- ZnO thin film-based fiber-optic gas sensors for carbon monoxide (CO) detection: Room temperature operating CO gas sensor using ZnO sensing film has been developed. The sensor operates based on the surface plasmon resonance (SPR) mechanism. This sensor has been reported to have a high sensitivity of 0.091/ppm and a fast response time of about 1 s towards a wide CO concentration range of 0.5–100 ppm at room temperature. These sensors are shown to selective towards CO, with a negligible interference with other gases such as NH, CO, NO, LPG and H. Therefore, ZnO thin film-based fiber-optic sensors have been shown as potential candidates for commercial applications of CO detection [66].
5.2. Photonic Crystal Gas Sensors
5.2.1. Structure and Mechanism
5.2.2. Fabrication
5.2.3. Applications
- Mesoporous Si-based photonic crystal gas sensors for VOC detection: Si-based photonic crystal have been researched to identify various VOCs by three differently sized patterns on one substrate. The patterns are etched on Si wafer by electrochemical anodization for mesopores with different sizes. The mesopores of 8 nm in diameter are produced in multilayer with 178, 229, and 300 nm of vertical spacings on Si substrate, which allows 430, 580, and 740 nm light to reflect. The analytes introduced onto this multi-layer-patterned PhC increases the effective refractive index and shifts the allowed wavelength of reflected light. Sensing performance of the device was tested with methanol, ethanol, and isopropanol in nitrogen carrier gas, which showed LOD in ppm range. In this method, analytes can be identified by measuring the wavelength shift gradient in time of each VOCs [68].
- Silica nano-sphere-based photonic crystal gas sensor: Self-assembled silica nano-spheres have been investigated to form photonic crystal for detection of water, ethanol and carbon disulfide (). The silica photonic crystal can be synthesized from silica colloid by drying the silica colloid on the substrate, followed by annealing at 600 C for sintering. This nano-structured silica can be coated with HKUST-1 to increase the interaction to analytes. The size of each silica nano-sphere is approximately 300 nm in diameter and arranged in face-centered cubic structure. Near infrared light can be introduced on [111] direction of silica nano-structure for gas-sensing test in the presence of analytes. Water, ethanol and tested for sensing performance have showed the response time at a few seconds with the estimated detection limit at 2.6 ppm for water, 0.3 ppm for ethanol and 0.5 ppm for [75].
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seiyama, T.; Kagawa, S. Study on a Detector for Gaseous Components Using Semiconductive Thin Films. Anal. Chem. 1966, 38, 1069–1073. [Google Scholar] [CrossRef]
- Qu, J.; Chai, Y.; Yang, S.X. A real-time de-noising algorithm for e-noses in a wireless sensor network. Sensors 2009, 9, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Refaat, T.F.; Ismail, S.; Koch, G.J.; Rubio, M.; Mack, T.L.; Notari, A.; Collins, J.E.; Lewis, J.; De Young, R.; Choi, Y.; et al. Backscatter 2-μm Lidar Validation for Atomospheric CO2 Differential Absorption Lidar Applications. IEEE Trans. Geosci. Remote Sens. 2011, 49, 572–580. [Google Scholar] [CrossRef]
- Donarelli, M.; Ottaviano, L. 2D Materials for Gas Sensing Applications: A Review on Graphene Oxide, MoS2, WS2 and Phosphorene. Sensors 2018, 18, 3638. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal oxide semi-conductor gas sensors in environmental monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed]
- Shankar, P.; Rayappan, J.B.B. Gas sensing mechanism of metal oxides: The role of ambient atmosphere, type of semiconductor and gases—A review. J. Sci. Lett. 2014, 4, 126. [Google Scholar]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Kakoty, P.; Bhuyan, M. Fabrication of Micromachined SnO2 Based MOS Gas Sensor with Inbuilt Microheater for Detection of Methanol. Sens. Transducers 2016, 204, 58–67. [Google Scholar]
- Li, W.; Zhao, D. An overview of the synthesis of ordered mesoporous materials. Chem. Commun. 2013, 49, 943–946. [Google Scholar] [CrossRef]
- Bochenkov, V.; Sergeev, G. Preparation and chemiresistive properties of nanostructured materials. Adv. Colloid Interface Sci. 2005, 116, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Schuler, M.; Helwig, N.; Schutze, A.; Sauerwald, T.; Ventura, G. Detecting trace-level concentrations of volatile organic compounds with metal oxide gas sensors. Proc. IEEE 2013, 3, 1–4. [Google Scholar]
- Lee, A.; Reedy, B. Temperature modulation in semiconductor gas sensing. Sens. Actuators B Chem. 1999, 60, 35–42. [Google Scholar] [CrossRef]
- Zhou, X.; Cheng, X.; Zhu, Y.; Elzatahry, A.A.; Alghamdi, A.; Deng, Y.; Zhao, D. Ordered porous metal oxide semiconductors for gas sensing. Chin. Chem. Lett. 2018, 29, 405–416. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Lonergan, M.; Severin, E.; Doleman, B.; Beaber, S.; Grubbs, R.; Lewis, N. Array-based vapor sensing using chemically sensitive carbon black- polymer resistors. Chem. Mater. 1996, 8, 2298–2312. [Google Scholar] [CrossRef]
- Ghoorchian, A.; Alizadeh, N. Chemiresistor gas sensor based on sulfonated dye-doped modified conducting polypyrrole film for high sensitive detection of 2, 4, 6-trinitrotoluene in air. Sens. Actuators B Chem. 2018, 255, 826–835. [Google Scholar] [CrossRef]
- MacDiarmid, A.G. “Synthetic metals”: A novel role for organic polymers (Nobel lecture). Angew. Chem. Int. Ed. 2001, 40, 2581–2590. [Google Scholar] [CrossRef]
- Liu, T.; Burger, C.; Chu, B. Nanofabrication in polymer matrices. Prog. Polym. Sci. 2003, 28, 5–26. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, B.; Majumdar, S. Polymers in sensor applications. Prog. Polym. Sci. 2004, 29, 699–766. [Google Scholar] [CrossRef]
- Bai, H.; Shi, G. Gas sensors based on conducting polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A survey on gas sensing technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef]
- Emadi, A.; Shafai, C.; Thomson, D.; Freund, M.; White, N.; Jayas, D. Polymer-based gas sensor on a thermally stable micro-cantilever. Procedia Eng. 2010, 5, 22–24. [Google Scholar] [CrossRef]
- Emadi, T.A.; Shafai, C.; Thomson, D.J.; Freund, M.S.; White, N.D.; Jayas, D.S. Polymer-based chemicapacitor sensor for 1-octanol and relative humidity detections at different temperatures and frequencies. IEEE Sens. J. 2013, 13, 519–527. [Google Scholar] [CrossRef]
- Carvalho, W.S.; Wei, M.; Ikpo, N.; Gao, Y.; Serpe, M.J. Polymer-Based Technologies for Sensing Applications. Anal. Chem. 2017, 90, 459–479. [Google Scholar] [CrossRef]
- Zhao, W.; Al-Nasser, L.F.; Shan, S.; Li, J.; Skeete, Z.; Kang, N.; Luo, J.; Lu, S.; Zhong, C.J.; Grausgruber, C.J.; et al. Detection of mixed volatile organic compounds and lung cancer breaths using chemiresistor arrays with crosslinked nanoparticle thin films. Sens. Actuators B Chem. 2016, 232, 292–299. [Google Scholar] [CrossRef] [Green Version]
- Zaporotskova, I.V.; Boroznina, N.P.; Parkhomenko, Y.N.; Kozhitov, L.V. Carbon nanotubes: Sensor properties. A review. Mod. Electron. Mater. 2016, 2, 95–105. [Google Scholar] [CrossRef]
- Szulczyński, B.; Gębicki, J. Currently commercially available chemical sensors employed for detection of volatile organic compounds in outdoor and indoor air. Environments 2017, 4, 21. [Google Scholar] [CrossRef]
- Pan, B.; Xing, B. Adsorption mechanisms of organic chemicals on carbon nanotubes. Environ. Sci. Technol. 2008, 42, 9005–9013. [Google Scholar] [CrossRef]
- Hou, Z.; Xu, D.; Cai, B. Ionization gas sensing in a microelectrode system with carbon nanotubes. Appl. Phys. Lett. 2006, 89, 213502. [Google Scholar] [CrossRef]
- Korotcenkov, G. Handbook of Gas Sensor Materials: Properties, Advantages and Shortcomings for Applications Volume 2: New Trends and Technologies; Springer: New York, NY, USA, 2014. [Google Scholar]
- Chopra, S.; McGuire, K.; Gothard, N.; Rao, A.; Pham, A. Selective gas detection using a carbon nanotube sensor. Appl. Phys. Lett. 2003, 83, 2280–2282. [Google Scholar] [CrossRef]
- Prasek, J.; Drbohlavova, J.; Chomoucka, J.; Hubalek, J.; Jasek, O.; Adam, V.; Kizek, R. Methods for carbon nanotubes synthesis. J. Mater. Chem. 2011, 21, 15872–15884. [Google Scholar] [CrossRef]
- Koziol, K.; Boskovic, B.O.; Yahya, N. Synthesis of Carbon Nanostructures by CVD Method. In Carbon and Oxide Nanostructures; Springer: Berlin/Heidelberg, Germany, 2010; pp. 23–49. [Google Scholar]
- Penza, M.; Martin, P.J.; Yeow, J.T. Carbon Nanotube Gas Sensors In Gas Sensing Fundamentals; Kohl, C.-D., Wagner, T., Eds.; Springer: Berlin, Germany, 2014; pp. 109–174. [Google Scholar]
- Bekyarova, E.; Davis, M.; Burch, T.; Itkis, M.; Zhao, B.; Sunshine, S.; Haddon, R. Chemically functionalized single-walled carbon nanotubes as ammonia sensors. J. Phys. Chem. B 2004, 108, 19717–19720. [Google Scholar] [CrossRef]
- Adjizian, J.J.; Leghrib, R.; Koos, A.A.; Suarez-Martinez, I.; Crossley, A.; Wagner, P.; Grobert, N.; Llobet, E.; Ewels, C.P. Boron-and nitrogen-doped multi-wall carbon nanotubes for gas detection. Carbon 2014, 66, 662–673. [Google Scholar] [CrossRef]
- Ballantine, D., Jr.; White, R.M.; Martin, S.J.; Ricco, A.J.; Zellers, E.; Frye, G.; Wohltjen, H. Acoustic Wave Sensors: Theory, Design and Physico-Chemical Applications; Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Sauerbrey, G. Use of quartz vibrator for weighting thin films on a microbalance. Zeitschrift fur Physik 1959, 155, 206–212. [Google Scholar] [CrossRef]
- Arshad, S.; Salleh, M.M.; Yahaya, M. Quartz Crystal Microbalance Gas Sensor for Detection of Volatile Organic Compounds using Titanium Dioxide coated with Dye-porphyrin. Solid State Sci. Technol. 2008, 16, 75–84. [Google Scholar]
- Hung, V.N.; Abe, T.; Minh, P.N.; Esashi, M. High-frequency one-chip multichannel quartz crystal microbalance fabricated by deep RIE. Sens. Actuators A Phys. 2003, 108, 91–96. [Google Scholar] [CrossRef]
- Hsueh, Y.-T.; Smith, R.L.; Northrup, M.A. A Microfabricated, Electrochemiluminescence Cell for the Detection of Amplified DNA . In Proceedings of the International Solid-State Sensors and Actuators Conference—TRANSDUCERS ’95, Stockholm, Sweden, 25–29 June 1995; pp. 768–771. [Google Scholar]
- Lakin, K.M.; Kline, G.R.; McCarron, K.T. High-Q microwave acoustic resonators and filters. IEEE Trans. Microw. Theory Tech. 1993, 41, 2139–2146. [Google Scholar] [CrossRef]
- Temel, F.; Tabakci, M. Calix [4] arene coated QCM sensors for detection of VOC emissions: Methylene chloride sensing studies. Talanta 2016, 153, 221–227. [Google Scholar] [CrossRef]
- Eslami, M.R.; Alizadeh, N. Ultrasensitive and selective QCM sensor for detection of trace amounts of nitroexplosive vapors in ambient air based on polypyrrole—Bromophenol blue nanostructure. Sens. Actuators B Chem. 2019, 278, 55–63. [Google Scholar] [CrossRef]
- Mujahid, A.; Dickert, F. Surface acoustic wave (SAW) for chemical sensing applications of recognition layers. Sensors 2017, 17, 2716. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.J.; Frye, G.C.; Senturia, S.D. Dynamics and response of polymer-coated surface acoustic wave devices: Effect of viscoelastic properties and film resonance. Anal. Chem. 1994, 66, 2201–2219. [Google Scholar] [CrossRef]
- Hamidon, M.N.; Skarda, V.; White, N.; Krispel, F.; Krempl, P.; Binhack, M.; Buff, W. Fabrication of high temperature surface acoustic wave devices for sensor applications. Sens. Actuators A Phys. 2005, 123, 403–407. [Google Scholar] [CrossRef] [Green Version]
- Urbańczyk, M.; Pustelny, T. The Application of Surface Acoustic Waves in Surface Semiconductor Investigations and Gas Sensors. In Modeling and Measurement Methods for Acoustic Waves and for Acoustic Microdevices; IntechOpen: London, UK, 2013. [Google Scholar] [Green Version]
- Emadi, T.A.; Buchanan, D.A. Design and Fabrication of a Novel MEMS Capacitive Transducer with Multiple Moving Membrane, M3-CMUT. IEEE Trans. Electron Devices 2014, 61, 890–896. [Google Scholar] [CrossRef]
- Park, K.K.; Lee, H.; Kupnik, M.; Oralkan, Ö.; Ramseyer, J.P.; Lang, H.P.; Hegner, M.; Gerber, C.; Khuri-Yakub, B.T. Capacitive micromachined ultrasonic transducer (CMUT) as a chemical sensor for DMMP detection. Sens. Actuators B Chem. 2011, 160, 1120–1127. [Google Scholar] [CrossRef]
- Ergun, A.S.; Yaralioglu, G.G.; Khuri-Yakub, B.T. Capacitive micromachined ultrasonic transducers: Theory and technology. J. Aerosp. Eng. 2003, 16, 76–84. [Google Scholar] [CrossRef]
- Lee, H.; Park, K.; Kupnik, M.; Oralkan, Ö.; Khuri-Yakub, B. Highly Sensitive Detection of DMMP Using a CMUT-Based Chemical Sensor. In Proceedings of the 2010 IEEE SENSORS, Kona, HI, USA, 1–4 November 2010; pp. 2122–2126. [Google Scholar]
- Park, K.K.; Lee, H.; Kupnik, M.; Khuri-Yakub, B.T. Fabrication of capacitive micromachined ultrasonic transducers via local oxidation and direct wafer bonding. J. Microelectromech. Syst. 2011, 20, 95–103. [Google Scholar] [CrossRef]
- Erguri, A.; Huang, Y.; Zhuang, X.; Oralkan, O.; Yarahoglu, G.G.; Khuri-Yakub, B.T. Capacitive micromachined ultrasonic transducers: Fabrication technology. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2005, 52, 2242–2258. [Google Scholar] [CrossRef]
- Emadi, A.; Buchanan, D. Capacitive Micromachined Ultrasonic Transducer with Multiple Deflectable Membranes. U.S. Patent 9,925,561, 27 March 2018. [Google Scholar]
- Gerardo, C.D.; Cretu, E.; Rohling, R. Fabrication and testing of polymer-based capacitive micromachined ultrasound transducers for medical imaging. Microsyst. Nanoeng. 2018, 4, 19. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, K.K.; Kupnik, M.; Khuri-Yakub, B.T. Functionalization layers for CO2 sensing using capacitive micromachined ultrasonic transducers. Sens. Actuators B Chem. 2012, 174, 87–93. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, K.K.; Kupnik, M.; Oralkan, O.; Khuri-Yakub, B.T. Chemical vapor detection using a capacitive micromachined ultrasonic transducer. Anal. Chem. 2011, 83, 9314–9320. [Google Scholar] [CrossRef]
- Geppert, T.M.; Schweizer, S.L.; Schilling, J.; Jamois, C.; Rhein, A.; Pergande, D.; Glatthaar, R.; Hahn, P.; Feisst, A.; Lambrecht, A.; et al. Photonic crystal gas sensors. Proc. SPIE 2004, 5511, 61–71. [Google Scholar] [CrossRef]
- Khan, M.R.R.; Kang, S.W. A high sensitivity and wide dynamic range fiber-optic sensor for low-concentration VOC gas detection. Sensors 2014, 14, 23321–23336. [Google Scholar] [CrossRef] [PubMed]
- Pal, B.P. Optical fiber sensors: A versatile technology platform for sensing. J. Indian Inst. Sci. 2014, 94, 283–310. [Google Scholar]
- Khan, M.R.R.; Kang, B.H.; Lee, S.W.; Kim, S.H.; Yeom, S.H.; Lee, S.H.; Kang, S.W. Fiber-optic multi-sensor array for detection of low concentration volatile organic compounds. Opt. Express 2013, 21, 20119–20130. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Fang, J.; Liang, Y.; Zhang, B.; Luo, Y.; Liu, X.; Li, Z.; Cai, X.; Xian, J.; Lin, H.; et al. All-fiber-optic VOC gas sensor based on side-polished fiber wavelength selectively coupled with cholesteric liquid crystal film. Sens. Actuators B Chem. 2018, 273, 1816–1826. [Google Scholar] [CrossRef]
- Narasimman, S.; Balakrishnan, L.; Meher, S.; Sivacoumar, R.; Alex, Z. ZnO nanoparticles based fiber optic gas sensor. AIP Conf. Proc. 2016, 1731, 050052. [Google Scholar]
- Paliwal, A.; Sharma, A.; Tomar, M.; Gupta, V. Carbon monoxide (CO) optical gas sensor based on ZnO thin films. Sens. Actuators B Chem. 2017, 250, 679–685. [Google Scholar] [CrossRef]
- Troia, B.; Paolicelli, A.; De Leonardis, F.; Passaro, V. Photonic crystals for optical sensing: A review. Adv. Photonic Cryst. 2013, 241–295. [Google Scholar] [CrossRef]
- Kelly, T.L.; Sega, A.G.; Sailor, M.J. Identification and quantification of organic vapors by time-resolved diffusion in stacked mesoporous photonic crystals. Nano Lett. 2011, 11, 3169–3173. [Google Scholar] [CrossRef]
- Zhang, Y.n.; Zhao, Y.; Lv, R.Q. A review for optical sensors based on photonic crystal cavities. Sens. Actuators A Phys. 2015, 233, 374–389. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Wu, P.; Zhu, C.; Elbaza, A.; Gu, Z.Z. Photonic crystal for gas sensing. J. Mater. Chem. C 2013, 1, 6086–6098. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.N.; Wang, Q. Research advances of photonic crystal gas and liquid sensors. Sens. Actuators B Chem. 2011, 160, 1288–1297. [Google Scholar] [CrossRef]
- Lin, H.; Zou, Y.; Hu, J. Double resonance 1-D glass-on-silicon photonic crystal cavities for single-molecule mid-infrared photothermal spectroscopy: Theory and design. In Proceedings of the 9th International Conference on Group IV Photonics (GFP), San Diego, CA, USA, 29–31 August 2012; pp. 132–134. [Google Scholar]
- Nair, R.V.; Vijaya, R. Photonic crystal sensors: An overview. Prog. Quantum Electron. 2010, 34, 89–134. [Google Scholar] [CrossRef]
- Ouyang, T.; Akbari-Sharbaf, A.; Park, J.; Bauld, R.; Cottam, M.G.; Fanchini, G. Self-assembled metallic nanoparticle superlattices on large-area graphene thin films: Growth and evanescent waveguiding properties. R. Soc. Chem. 2015, 5, 98814–98821. [Google Scholar] [CrossRef]
- Lu, G.; Farha, O.K.; Kreno, L.E.; Schoenecker, P.M.; Walton, K.S.; Van Duyne, R.P.; Hupp, J.T. Fabrication of metal-organic framework-containing silica-colloidal crystals for vapor sensing. Adv. Mater. 2011, 23, 4449–4452. [Google Scholar] [CrossRef]












© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced Micro- and Nano-Gas Sensor Technology: A Review. Sensors 2019, 19, 1285. https://doi.org/10.3390/s19061285
Nazemi H, Joseph A, Park J, Emadi A. Advanced Micro- and Nano-Gas Sensor Technology: A Review. Sensors. 2019; 19(6):1285. https://doi.org/10.3390/s19061285
Chicago/Turabian StyleNazemi, Haleh, Aashish Joseph, Jaewoo Park, and Arezoo Emadi. 2019. "Advanced Micro- and Nano-Gas Sensor Technology: A Review" Sensors 19, no. 6: 1285. https://doi.org/10.3390/s19061285
APA StyleNazemi, H., Joseph, A., Park, J., & Emadi, A. (2019). Advanced Micro- and Nano-Gas Sensor Technology: A Review. Sensors, 19(6), 1285. https://doi.org/10.3390/s19061285



