Rapid Identification of Kudzu Powder of Different Origins Using Laser-Induced Breakdown Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Experimental Materials
2.3. Data Acquisition
2.4. Selection of Characteristic Wavelength
2.5. Discriminant Analysis Method
3. Results and Discussion
3.1. Spectral Analysis of Different Samples
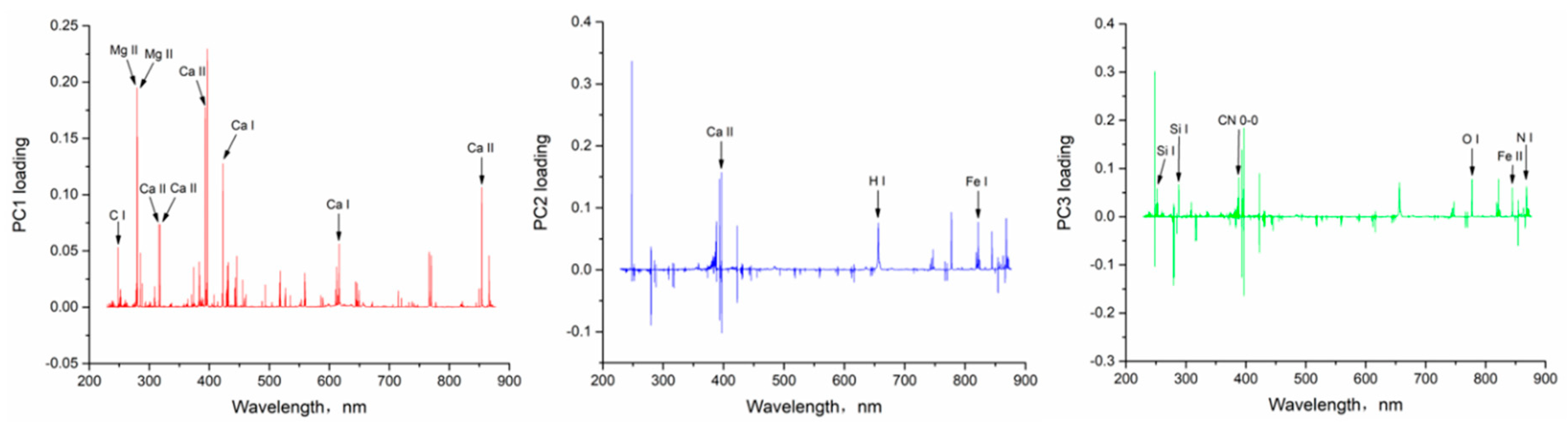
3.2. Principal Component Analysis
3.3. Discriminant Analysis Model Based on Full Spectrum
3.4. Selection of Characteristic Spectral Lines
3.5. Discriminant Analysis Based on Characteristic Wavelength
4. Discussion
- The original spectra of different samples were acquired by LIBS. Principal component analysis was performed and the first 3 principal components had a total variance of 99.55%.
- The accuracy of discriminant models based on the full spectrum, including ELM, SIMCA, KNN and RF, was more than 99.00%. The prediction results of KNN and RF models were best, of which the accuracy of calibration and prediction sets of kudzu powder from different producing areas both reached 100%. The results showed that LIBS spectroscopy is feasible for rapid identification of kudzu powder from different producing areas.
- The accuracy of discriminant models based on 18 characteristic wavelengths, including ELM, SIMCA, KNN and RF, was more than 98.00%. Random forest and KNN have the same excellent identification results, and the accuracy of calibration and prediction sets of kudzu powder from different producing areas reached 100%. Compared with the full spectrum discriminant analysis model, the discriminant analysis model based on the characteristic wavelength had almost the same discriminant effects, and the input variables were reduced by 99.92%. The results of the research showed that the characteristic wavelength could be used instead of the LIBS full spectrum to quickly identify kudzu powder from different producing areas, which had the advantages of reducing input, simplifying the model, increasing the speed and improving the model effect.
Author Contributions
Funding
Conflicts of Interest
References
- Su, F.M.; Chang, J.S.; Wang, K.C.; Tsai, J.J.; Chiang, L.C. A water extract of Pueraria lobata inhibited cytotoxicity of enterovirus 71 in a human foreskin fibroblast cell line. Kaohsiung J. Med Sci. 2008, 24, 523–530. [Google Scholar] [CrossRef]
- Van Hung, P.; Morita, N. Chemical compositions, fine structure and physicochemical properties of kudzu (Pueraria lobata) starches from different regions. Food Chem. 2007, 105, 749–755. [Google Scholar] [CrossRef]
- Keung, W.M.; Vallee, B.L. Kudzu root: An ancient Chinese source of modern antidipsotropic agents. Phytochemistry 1998, 47, 499–506. [Google Scholar] [CrossRef]
- Xu, L.; Shi, W.; Cai, C.B.; Zhong, W.; Tu, K. Rapid and nondestructive detection of multiple adulterants in kudzu starch by near infrared (NIR) spectroscopy and chemometrics. LWT-Food Sci. Technol. 2015, 61, 590–595. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, H.; Yang, H.L.; Nie, L.; Zang, H.C. Rapid determination of major bioactive isoflavonoid compounds during the extraction process of kudzu (Pueraria lobata) by near-infrared transmission spectroscopy. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2015, 137, 1403–1408. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, H.; Cheng, M.; Cao, S.; Qiao, M.; Zhang, B.; Ding, L.; Qiu, F. New hepatoprotective isoflavone glucosides from Pueraria lobata (Willd.) Ohwi. Nat. Prod. Res. 2018. [Google Scholar] [CrossRef]
- Zhao, C.X.; Chan, H.Y.; Yuan, D.L.; Liang, Y.Z.; Lau, T.Y.; Chau, F.T. Rapid simultaneous determination of major isoflavones of Pueraria lobata and discriminative analysis of its geographical origins by principal component analysis. Phytochem. Anal. 2011, 22, 503–508. [Google Scholar] [CrossRef]
- Ahmed, T.; Javed, S.; Tariq, A.; Budzynska, B.; D’Onofrio, G.; Daglia, M.; Nabavi, S.F.; Nabavi, S.M. Daidzein and its effects on brain. Curr. Med. Chem. 2017, 24, 365–375. [Google Scholar] [PubMed]
- Das, D.; Sarkar, S.; Bordoloi, J.; Wann, S.B.; Kalita, J.; Manna, P. Daidzein, its effects on impaired glucose and lipid metabolism and vascular inflammation associated with type 2 diabetes. Biofactors 2018, 44, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Barnes, S.; Prasain, J.; D’Alessandro, T.; Arabshahi, A.; Botting, N.; Lila, M.A.; Jackson, G.; Janle, E.M.; Weaver, C.M. The metabolism and analysis of isoflavones and other dietary polyphenols in foods and biological systems. Food Funct. 2011, 2, 235–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delmonte, P.; Rader, J.I. Analysis of isoflavones in foods and dietary supplements. J. Aoac Int. 2006, 89, 1138–1146. [Google Scholar] [PubMed]
- Wei, S.-Y.; Chen, Y.; Xu, X.-Y. Progress on the pharmacological research of puerarin: A review. Chin. J. Nat. Med. 2014, 12, 407–414. [Google Scholar] [CrossRef]
- Zhang, Z.; Lam, T.-N.; Zuo, Z. Radix Puerariae: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J. Clin. Pharmacol. 2013, 53, 787–811. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Li, G.Q.; Li, K.M.; Razmovski-Naumovski, V.; Chan, K. Kudzu root: Traditional uses and potential medicinal benefits in diabetes and cardiovascular diseases. J. Ethnopharmacol. 2011, 134, 584–607. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-X.; Zhang, H.; Peng, C. Puerarin: A review of pharmacological effects. Phytother. Res. 2014, 28, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Tam, W.Y.; Chook, P.; Qiao, M.; Chan, L.T.; Chan, T.Y.K.; Poon, Y.K.; Fung, K.P.; Leung, P.C.; Woo, K.S. The efficacy and tolerability of adjunctive alternative herbal medicine (Salvia miltiorrhiza and Pueraria lobata) on vascular function and structure in coronary patients. J. Altern. Complement. Med. 2009, 15, 415–421. [Google Scholar] [CrossRef]
- Lee, J.S. Supplementation of Pueraria radix water extract on changes of antioxidant enzymes and lipid profile in ethanol-treated rats. Clin. Chim. Acta 2004, 347, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.W.; Chen, S.M.; Fu, O.Y.; Yang, I.C.; Tsai, C.Y. A robust identification model for herbal medicine using near infrared spectroscopy and artificial neural network. J. Food Drug Anal. 2011, 19, 9–17. [Google Scholar]
- Hu, L.Q.; Ma, S.; Yin, C.L. Discrimination of geographical origin and detection of adulteration of kudzu root by fluorescence spectroscopy coupled with multi-way pattern recognition. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2018, 193, 87–94. [Google Scholar] [CrossRef]
- Han, M.X.; Zhou, Q.; Li, Q.H.; Sun, S.Q. Identification of three-step IR spectra of Radix Puerariae from sifferent habitats. Spectrosc. Spectr. Anal. 2009, 29, 1851–1855. [Google Scholar]
- Liu, F.; Ye, L.; Peng, J.; Song, K.; Shen, T.; Zhang, C.; He, Y. Fast detection of copper content in rice by laser-induced breakdown spectroscopy with uni- and multivariate analysis. Sensors 2018, 18, 705. [Google Scholar] [CrossRef]
- Peng, J.; Liu, F.; Zhou, F.; Song, K.; Zhang, C.; Ye, L.; He, Y. Challenging applications for multi-element analysis by laser-induced breakdown spectroscopy in agriculture: A review. Trends Anal. Chem. 2016, 85, 260–272. [Google Scholar] [CrossRef]
- Peng, J.; He, Y.; Ye, L.; Shen, T.; Liu, F.; Kong, W.; Liu, X.; Zhao, Y. Moisture influence reducing method for heavy metals detection in plant materials using laser-induced breakdown spectroscopy: A case study for chromium content detection in rice leaves. Anal. Chem. 2017, 89, 7593–7600. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Altuwiriqi, R.; Gondal, M.A.; Aldakheel, R.K.; Alotaibi, H.F. Qualitative and quantitative analysis of human nails to find correlation between nutrients and vitamin D deficiency using LIBS and ICP-AES. Talanta 2018, 185, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Liu, F.; Shen, T.; Ye, L.; Kong, W.; Wang, W.; Liu, X.; He, Y. Comparative study of the detection of chromium content in rice leaves by 532 nm and 1064 nm laser-induced breakdown spectroscopy. Sensors 2018, 18, 621. [Google Scholar] [CrossRef]
- De Giacomo, A. A novel approach to elemental analysis by laser induced breakdown spectroscopy based on direct correlation between the electron impact excitation cross section and the optical emission intensity. Spectrochim. Acta Part B-At. Spectrosc. 2011, 66, 661–670. [Google Scholar] [CrossRef]
- Costa, V.C.; Babos, D.V.D.; Aquino, F.W.B.D.; Virgílio, A.; Amorim, F.A.C.; Pereira-Filho, E.R. Direct determination of Ca, K and Mg in cassava flour samples by laser-induced breakdown spectroscopy (LIBS). Food Anal. Methods 2018, 11, 1886–1896. [Google Scholar] [CrossRef]
- Carvalho, G.G.A.A.D.; Guerra, M.B.B.; Adame, A.; Nomura, C.S.; Krug, F.J. Recent advances in LIBS and XRF for the analysis of plants. J. Anal. At. Spectrom. 2018, 33, 911–1094. [Google Scholar]
- Zhang, C.; Shen, T.; Liu, F.; He, Y. Identification of coffee varieties using laser-induced breakdown spectroscopy and chemometrics. Sensors 2018, 18, 95. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.W.; Tang, S.S.; Ma, S.X.; Ma, Y.Y.; Ha, Z.Q.; Gu, Y.M.; Gu, L.B.; Lu, Y.F.; Zeng, X.Y. Accuracy and stability improvement for meat species identification using multiplicative scatter correction and laser-induced breakdown spectroscopy. Opt. Express 2018, 26, 10119–10127. [Google Scholar] [CrossRef]
- Kim, D.; Yang, J.; Choi, S.J.; Yoh, J. Express: Analytical methods to distinguish the positive and negative spectra of mineral and environmental elements using deep ablation laser-induced breakdown spectroscopy (LIBS). Appl. Spectrosc. 2018, 72, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Umar, Z.A.; Ahmed, N.; Ahmed, R.; Liaqat, U.; Baig, M.A. Elemental composition analysis of granite rocks using LIBS and LA-TOF-MS. Appl. Opt. 2018, 57, 4985–4991. [Google Scholar] [CrossRef]
- Noll, R.; Frickebegemann, C.; Connemann, S.; Meinhardt, C.; Sturm, V. LIBS analyses for industrial applications—An overview of developments from 2014 to 2018. J. Anal. At. Spectrom. 2018, 33, 945–956. [Google Scholar] [CrossRef]
- Wang, J.M.; Liao, X.Y.; Zheng, P.C.; Xue, S.W.; Peng, R. Classification of Chinese herbal medicine by laser-induced breakdown spectroscopy with principal component analysis and artificial neural network. Anal. Lett. 2018, 51, 575–586. [Google Scholar] [CrossRef]
- Wang, J.M.; Xue, S.W.; Zheng, P.C.; Chen, Y.Y.; Peng, R. Determination of lead and copper in Ligusticum wallichii by laser-induced breakdown spectroscopy. Anal. Lett. 2017, 50, 2000–2011. [Google Scholar] [CrossRef]
- Hu, L.Q.; Yin, C.L. Fourier transform infrared spectroscopy coupled with chemometrics for determining the geographical origin of kudzu root and the detection and quantification of adulterants in kudzu root. Anal. Methods 2017, 9, 3643–3652. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, C.; Liu, F.; Kong, W.; He, Y.; Lou, B. Hyperspectral imaging analysis for ripeness evaluation of strawberry with support vector machine. J. Food Eng. 2016, 179, 11–18. [Google Scholar] [CrossRef]
- Xie, C.; Shao, Y.; Li, X.; He, Y. Detection of early blight and late blight diseases on tomato leaves using hyperspectral imaging. Sci. Rep. 2015, 5, 16564. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Wu, Z.; Su, R.; Ruan, G.; Du, F.; Li, G. Current application of chemometrics in traditional Chinese herbal medicine research. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1026, 27–35. [Google Scholar] [CrossRef]
- Huang, G.; Huang, G.B.; Song, S.; You, K. Trends in extreme learning machines: A review. Neural Netw. 2015, 61, 32–48. [Google Scholar] [CrossRef]
- Huang, G.B.; Zhu, Q.Y.; Siew, C.K. Extreme learning machine: Theory and applications. Neurocomputing 2006, 70, 489–501. [Google Scholar] [CrossRef] [Green Version]
- Shamshirband, S.; Mohammadi, K.; Yee, P.L.; Petkovic, D.; Mostafaeipour, A. A comparative evaluation for identifying the suitability of extreme learning machine to predict horizontal global solar radiation. Renew. Sustain. Energy Rev. 2015, 52, 1031–1042. [Google Scholar] [CrossRef]
- Jiang, H.; Zhu, W. Determination of pear internal quality attributes by Fourier transform near infrared (FT-NIR) spectroscopy and multivariate analysis. Food Anal. Methods 2013, 6, 569–577. [Google Scholar] [CrossRef]
- Strobl, C.; Malley, J.; Tutz, G. An introduction to recursive partitioning: Rationale, application, and characteristics of classification and regression trees, bagging, and random forests. Psychol. Methods 2009, 14, 323–348. [Google Scholar] [CrossRef] [PubMed]
- Forina, M.; Oliveri, P.; Lanteri, S.; Casale, M. Class-modeling techniques, classic and new, for old and new problems. Chemom. Intell. Lab. Syst. 2008, 93, 132–148. [Google Scholar] [CrossRef]
- Moncayo, S.; Manzoor, S.; Navarro-Villoslada, F.; Caceres, J.O. Evaluation of supervised chemometric methods for sample classification by laser induced breakdown spectroscopy. Chemom. Intell. Lab. Syst. 2015, 146, 354–364. [Google Scholar] [CrossRef]
- Fernandez-Bravo, A.; Delgado, T.; Lucena, P.; Javier Laserna, J. Vibrational emission analysis of the CN molecules in laser-induced breakdown spectroscopy of organic compounds. Spectrochim. Acta Part B-At. Spectrosc. 2013, 89, 77–83. [Google Scholar] [CrossRef]



| Discriminant Analysis Model | Parameter [a] | Accuracy of Calibration Set | Accuracy of Prediction Set |
|---|---|---|---|
| ELM | 53 | 100% | 99.30% |
| SIMCA | (7,12,11,8,2) | 100% | 99.00% |
| KNN | 3 | 100% | 100% |
| RF | (151,7) | 100% | 100% |
| Number | Wavelength (nm) | Element | Number | Wavelength (nm) | Element |
|---|---|---|---|---|---|
| 1 | 247.86 | C I | 10 | 396.85 | Ca II |
| 2 | 251.61 | Si I | 11 | 422.67 | Ca I |
| 3 | 279.55 | Mg II | 12 | 616.38 | Ca I |
| 4 | 280.27 | Mg II | 13 | 656.28 | H I |
| 5 | 288.15 | Si I | 14 | 777.54 | O I |
| 6 | 315.89 | Ca II | 15 | 821.58 | Fe I |
| 7 | 317.93 | Ca II | 16 | 844.80 | Fe II |
| 8 | 388.22 | CN 0-0 | 17 | 854.21 | Ca II |
| 9 | 393.37 | Ca II | 18 | 868.02 | N I |
| Discriminant Analysis Model | Parameter | Accuracy of Calibration Set | Accuracy of Prediction Set |
|---|---|---|---|
| ELM | 118 | 100% | 99.30% |
| SIMCA | (3,4,3,3,2) | 100% | 98.00% |
| KNN | 3 | 100% | 100% |
| RF | (151,7) | 100% | 100% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Wang, W.; Shen, T.; Peng, J.; Kong, W. Rapid Identification of Kudzu Powder of Different Origins Using Laser-Induced Breakdown Spectroscopy. Sensors 2019, 19, 1453. https://doi.org/10.3390/s19061453
Liu F, Wang W, Shen T, Peng J, Kong W. Rapid Identification of Kudzu Powder of Different Origins Using Laser-Induced Breakdown Spectroscopy. Sensors. 2019; 19(6):1453. https://doi.org/10.3390/s19061453
Chicago/Turabian StyleLiu, Fei, Wei Wang, Tingting Shen, Jiyu Peng, and Wenwen Kong. 2019. "Rapid Identification of Kudzu Powder of Different Origins Using Laser-Induced Breakdown Spectroscopy" Sensors 19, no. 6: 1453. https://doi.org/10.3390/s19061453
APA StyleLiu, F., Wang, W., Shen, T., Peng, J., & Kong, W. (2019). Rapid Identification of Kudzu Powder of Different Origins Using Laser-Induced Breakdown Spectroscopy. Sensors, 19(6), 1453. https://doi.org/10.3390/s19061453






