1. Introduction
Methane (CH
4) is one of the main anthropogenic greenhouse gases in the atmosphere. Its concentration has increased up to 1.87 ppm, starting from a value of 715 ppb in preindustrial times [
1]. Due to the effect of CH
4 on global warming and climate change, methane detection is mandatory to monitor variations in atmospheric concentration as well as identify its main sources. These objectives can be fulfilled by real-time and in situ measurements of CH
4 concentration in the atmosphere. Thus, a methane sensor must guarantee the following: (i) high sensitivity in the sub-part-per-million range; (ii) high selectivity to discriminate the CH
4 signal from other gas components in the atmosphere; and (iii) fast response time to track any variation in concentration. Moreover, robustness, compactness, and insensibility to environmental external noise are required for in-field operation. Various types of sensors have been developed to detect methane in the atmosphere, showing different advantages and disadvantages in terms of selectivity, sensitivity, response time, robustness, and stability for prolonged measurements. A common issue to be addressed is the presence of water in air, the change in humidity of which affects the performance of sensors. Semiconductor sensors based on metal oxide film are robust and long-lived but not suitable for in-field detection of methane. They require high power consumption to keep the metal oxide at the 100–500 °C temperature range, are sensible to variations in temperature and humidity, and suffer from poor selectivity. In [
2], a CH
4 semiconductor sensor was tested with a measurement of the lab air for 31 days, showing an accuracy of the order of 0.8–2.7 ppm, which precluded its application for atmospheric CH
4 monitoring. Nondispersive infrared spectroscopy (NDIR) sensors do not have the sensitivity level required for environmental application. In addition, they suffer from spectral interference by water, and the use of optical filters requires a dry ambience because water condensation causes a variation in the transmittance efficiency. Laser absorption spectroscopy (LAS) employs a laser as an excitation source to improve detection selectivity, overcoming the need for optical filters used in NDIR. It offers high detection sensitivity thanks to the exploitation of multipass gas cells to increase the molecule absorption path length. In [
3], a multipass cell was used in a LAS-based sensor to detect atmospheric methane, reaching a detection sensitivity of 100 ppb. Measurements of CH
4 in ambient air were performed for two days using a dryer and a particle filter to remove humidity. A cavity ring-down spectroscopy (CRDS) sensor was developed by Picarro for the detection of CH
4, H
2O, and CO
2 in the atmosphere, reaching a CH
4 minimum detection limit of less than 1 ppb for an integration time of 5 s [
4]. In [
5], a comparison of portable devices for the detection of methane for soil research was reported. The performance of LAS and Fourier transform infrared spectroscopy (FTIR) sensors were tested, showing detection limits of 0.01 ppm with 10 s response time and 0.053 ppm with less than 120 s response time, respectively. A review and comparison of optical sensors for CH
4 detection is reported in [
6], even though most of the sensors have not been tested with prolonged measurements of CH
4 in ambient air. Among LAS sensors [
7,
8,
9], QEPAS sensors were found to fulfil all the needed requirements, providing highly sensitive measurements of CH
4 [
10,
11,
12]. QEPAS is based on the absorption of modulated laser light by the target gas. The laser beam is focused between the prongs of a quartz tuning fork (QTF) at one of the antinode points of the QTF vibrational mode and is modulated at the associated resonance frequency or at one of its subharmonics. The energy of the excited roto-vibrational states is released via inelastic collisions among the surrounding molecules, generating a pressure wave. The pressure wave is detected by the quartz tuning fork, acting as a transducer of the prongs’ mechanical deflection induced by the pressure wave, into an electrical signal thanks to the piezoelectricity of the quartz [
13]. The QTF is acoustically coupled with microresonator tubes to amplify the sound wave [
14]. The generation of the acoustic wave, and consequently of the QEPAS signal, depends on the relaxation rate of the excited molecules’ vibrational energy into the kinetic energy (translation) of the surrounding molecules (VT relaxation). This effect has been investigated in several studies [
15,
16], and it has become particularly relevant for detection of gas species with slow VT relaxation rates, such as CH
4. In the latter case, a laser modulation frequency lower than the effective analyte relaxation rate in the gas matrix can be selected in order to allow a complete release of the absorbed energy between consecutive optical excitations. This guarantees a highly efficient sound wave generation. The development of custom QTFs with resonance frequencies lower than 20 kHz [
17] was aimed to address this issue. However, the molecule relaxation rate depends on the gas matrix, and a variation in the matrix composition can affect the QEPAS signal, particularly in the case of slow relaxing gases.
Once the gas target molecules are excited, they can relax through different channels via collisions with any kind of molecule composing the mixture. The relaxation rate of the target molecule in a matrix is then provided by the sum of the relaxation rates characterizing every possible energy transfer pathway, weighted by the concentration of each species in the mixture. In particular, for environmental monitoring application, CH
4 is detected in a standard air-like matrix containing water vapor in the concentration range of a few percentage points. The required CH
4 and H
2O concentration range for environmental applications are 0.1–1000 ppm and from 100 ppm to 3%, respectively. The influence of water on the performance of QEPAS methane sensors has been investigated in several studies [
18,
19]. The water vapor molecules act as promoters for the VT relaxation processes of CH
4. As a result, a variation of water concentration in air causes a variation in the QEPAS methane signal not related to a change in the CH
4 concentration. In order to guarantee reliable CH
4 concentration measurement, two sensor configurations can be implemented. The first one involves adding a humidifier in series to the gas delivery line to fix the H
2O concentration [
20,
21,
22] and calibrate the sensor. The benefit is enhancement of the CH
4 signal due to the presence of constant H
2O concentration, resulting in higher CH
4 detection sensitivity. On the other hand, a periodic check on the humidifier quality is mandatory. For example, a constant humidity of the gas mixture can be achieved by flowing the gas through a Nafion membrane humidifier immersed in a temperature-controlled water bath, which requires periodic refilling. The second approach consists of measuring the H
2O and CH
4 concentrations simultaneously using a multigas detection scheme and properly adjusting the CH
4 QEPAS signal with respect to the H
2O signal. In this case, either multiple laser sources operating simultaneously can be employed or a single laser source whose spectral range covers both water and methane absorption lines [
23,
24]. In [
24], the atmospheric detection of N
2O, CH
4, and H
2O was achieved using a single QCL whose spectral range covers absorption lines of all three gases. In [
4], atmospheric measurements of CH
4, CO
2, and H
2O were performed using a cavity ring-down analyzer consisting of two lasers alternatively selected using an optical switch. In [
25], a QEPAS sensor based on a frequency division multiplexing scheme with a single QTF and two laser sources was implemented to simultaneously detect H
2O and CH
4. In that case, the water signal was used to compensate the influence of H
2O on the CH
4 signal. However, using a single laser source does not allow a simultaneous detection of both gas species and an instantaneous calibration; on the other hand, using multilaser sources increases the complexity of the experimental apparatus, making the sensor less suitable for in-field applications.
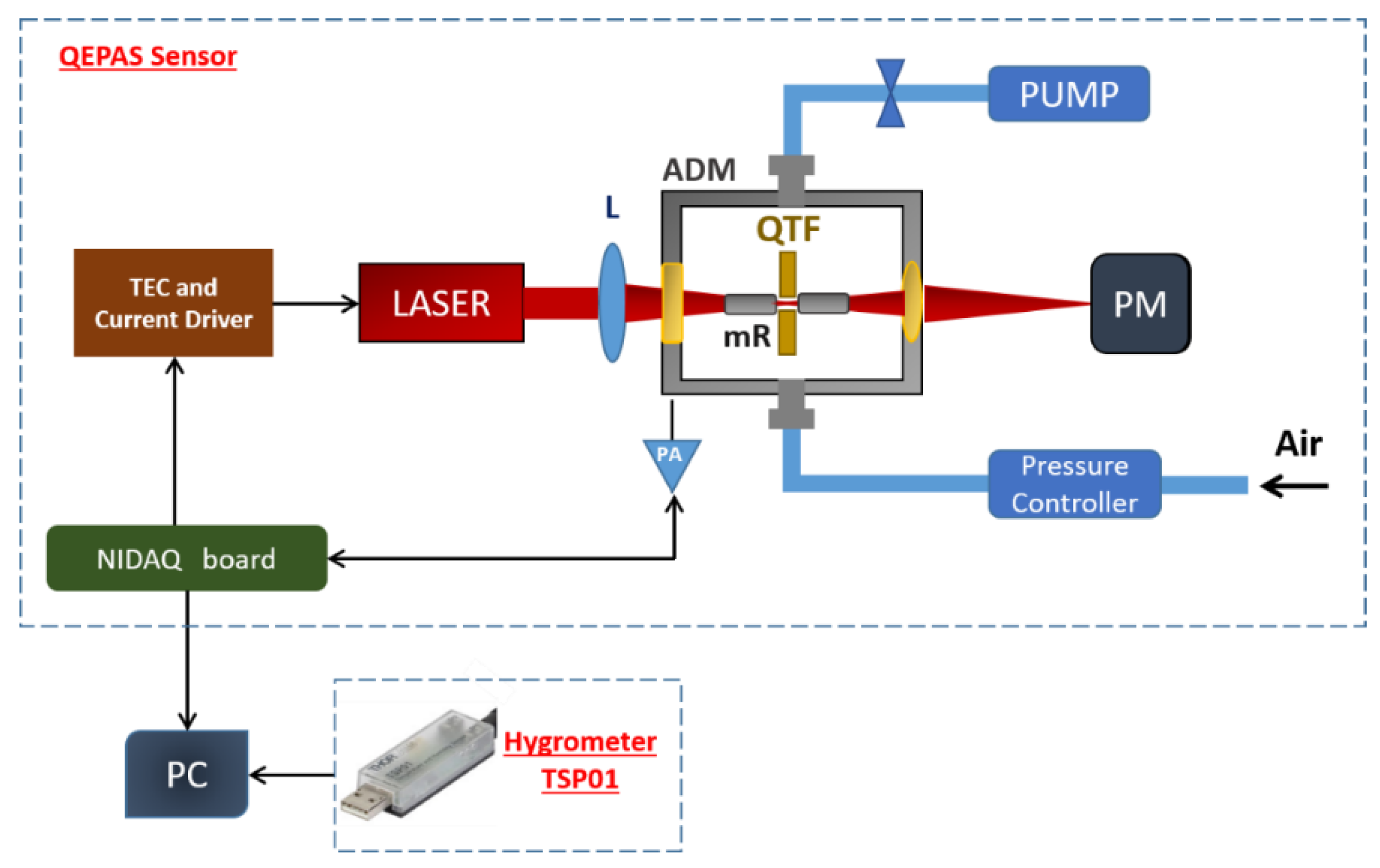
In this work, we demonstrated that for the environmental monitoring of CH4, a methane QEPAS sensor can be used in combination with an electronic hygrometer monitoring the variation of water vapor in the air. For comparison, the water signal was also detected using the same QEPAS sensor, targeting a water absorption line nearby the methane one. The accuracy and precision of the H2O concentration measurements provided by the hygrometer allows compensating the water influence on CH4 QEPAS signal.
3. Measurements of CH4 and H2O in Air
Figure 2 shows comparison between the absorption cross section of standard air at 200 Torr simulated using the HITRAN database [
26] (
Figure 2a) and a representative QEPAS scan of the laboratory air (
Figure 2b).
From comparison, the highest peak at 232.35 mA clearly corresponds to the water absorption line, while the lower peak at 229.25 mA corresponds to the methane line. A preliminary calibration of the QEPAS sensor for the detection of CH
4 in dry N
2 was performed. The sensor was calibrated by acquiring the QEPAS signal of the CH
4 absorption line at 2988.8 cm
−1 for different concentrations of CH
4. The mixtures were obtained starting from a certified concentration of 45 ppm of CH
4, which was diluted in pure N
2 by means of a gas mixer. By linearly fitting the peak values as a function of the CH
4 concentration, the calibration curve y = (1.07 mV/ppm)
x was obtained with an R
2 = 0.999, confirming the linearity of the sensor response with respect to the CH
4 concentration. A minimum detection limit of ~180 ppb was achieved for a 1σ noise of 0.20 mV at 200 ms integration time. We performed an Allan variance analysis [
27] of the hygrometer TSP01 signal to study the long-term stability of the absolute humidity measurement. The TSP01 sensor was closed in a climate chamber to fix the temperature and the relative humidity of surrounding air at 27 °C and 40%, respectively. The TSP01 signal was acquired for ~4 h with an integration time of 2 s. The absolute humidity is the total mass of water vapor present in a certain volume or mass of air. It gives a measurement of the concentration of water vapor in air. The relative humidity is the ratio between the amount of water vapor in air and the amount of water vapor that would saturate the air at the same temperature and pressure. The H
2O QEPAS signal is a measurement of the absolute humidity; TSP01 measures the relative humidity and the temperature of air. Both temperature (
T) and relative humidity (
RH) values were used to calculate the absolute humidity (
AH) of the air sample, in ppm using the following equations [
28,
29]:
where
P is the ambient pressure (760 Torr),
Pw is the water vapor pressure, and
Pws is the saturated water vapor pressure. In
Figure 3, the Allan deviation of the absolute humidity signal (calculated with Equation (1) using the relative humidity and the temperature measured by TSP01) is shown as a function of the signal integration time.
The Allan deviation slightly increased from 2 to 20 s and then followed the √t dependence expected, where the dominant noise source was the flicker noise. The accuracy of temperature and relative humidity measurements were 0.5 °C and 2%, respectively, as reported in the datasheet of the instrument. The precision of TSP01, evaluated experimentally with prolonged measurements of T and RH at fixed condition, was 0.01 °C for temperature and 0.1% for relative humidity. These values determined an accuracy and a precision on the calculated absolute humidity of ~350 and ~30 ppm, respectively, estimated using the error propagation for Equation (1).
An investigation of the long-term stability of the methane peak values was performed when no water vapor was in the gas line. With this aim, a 10 h long measurement of fixed 45 ppm CH
4 concentration in dry N
2 was carried out. The measurements were performed by acquiring QEPAS spectral scans of the CH
4 absorption line with a 200 ms integration time and by extracting the peak value from each scan. Similarly, an 8 h long measurement of fixed concentration of H
2O was performed to test the long-term stability of the H
2O QEPAS peak values. A PermSelect humidifier was inserted in the gas line upstream the ADM to keep water concentration fixed to 1.6%. The CH
4 and the H
2O QEPAS peak values are reported in
Figure 4 as a function of time.
Both the CH
4 and H
2O QEPAS signals had no appreciable drifts during 10 h of continuous measurement. The 1σ value of fluctuations was 0.20 mV for CH
4 and 0.22 mV for H
2O, confirming the long-term stability of the sensor when both gases are detected separately. Once the sensor was calibrated, the CH
4 and H
2O concentrations in laboratory ambient air were continuously monitored for 62 h over a weekend. The CH
4 signal was measured using the QEPAS sensor, while the H
2O signal was acquired using both the QEPAS sensor and the hygrometer TSP01. ICL wavelength shifts can affect the QEPAS measurement. This is expected when the laser line is fixed to the CH
4 absorption peak without a line-locking feedback system. To avoid this issue, QEPAS spectral scans were acquired by setting the temperature of the ICL to 25 °C and scanning the laser current in the range 228–234 mA, to detect both CH
4 and H
2O (see
Figure 2b) absorption lines. The pressure and the flow of the sample air flushed through the ADM were set to 200 Torr and 25 standard cubic centimeter per minute (sccm), respectively. For each spectral scan, the QEPAS peak signals of H
2O and CH
4 absorption features were extracted and are plotted as a function of time in
Figure 5a,b, respectively. The time interval between two consecutive peaks of the same gas species was 3.8 min. Simultaneously, the TSP01 sensor was placed close to the QEPAS sensor to acquire the temperature and the relative humidity of the laboratory ambient air. The temperature and relative humidity excursion intervals recorded during 62 h of sensor operation were 24–28 °C and 32–44%, respectively. The absolute humidity is plotted in
Figure 5c as a function of time.
Figure 5d reports the standardized CH
4 QEPAS, H
2O QEPAS, and the absolute humidity signals, overlapped on the same
x-axis; each standardized signal was obtained by subtracting from the original signal its mean and dividing the difference by the standard deviation. The three signals exhibited the same trend as a function of time. Thus, the QEPAS sensor and the electronic hygrometer detected the same water vapor variations in ambient air, which in turn affected the CH
4 signal. Shifts of QTF resonance frequency as well as variations in the Q factor can affect QEPAS measurements. Indeed, the resonance frequency of the QTF determines the modulation frequency applied to the laser current, while the QEPAS signal is proportional to the Q factor itself. At the beginning and at the end of the 62 h long series of measurements, the QTF resonance curve was acquired, and no appreciable shifts in the frequency or sensible variations of the Q factor were measured.
As a first step, the absolute humidity values measured by TSP01 can be used to calibrate the QEPAS sensor for water vapor detection. In
Figure 6, the H
2O QEPAS signal is plotted as a function of the absolute humidity (blue squares).
The deviations of the H
2O QEPAS signals with respect to hygrometer measurements can be mainly ascribed to a different precision of the two acquisition techniques and to the fact that the QTF is located in the gas cell, while the hygrometer is placed in the outside environment. The most immediate technique for smoothing signals consisting of equidistant points is the moving average. With a fixed subset size, the first element of the moving average is obtained by taking the average of the initial fixed subset of the number series. Then, the subset is modified by excluding the first datum of the series and including the next value in the subset. A LabVIEW-based software was implemented to perform a moving average and at the same time establish the optimized size of subsets. Starting from datasets with two points, a linear fit was performed on the obtained “smoothed” signal, and the R
2 value was extracted. Then, the subset size was increased, and R
2 values were plotted as a function of the subset size. We observed that R
2 value rapidly rose as the subset size increased until a plateau value of 0.99 was reached. This condition was obtained when the subset size was 20.
Figure 6 shows the averaged dataset when the subset size was 20 (green circles) and the best linear fit (red line), which returned a slope of
k1 = 5.9 μV/ppm and a negligible intercept. This curve can be used as a calibration curve to convert the H
2O QEPAS signal into water vapor concentration.
Figure 5d shows the influence of H
2O variations on the CH
4 QEPAS signal due to water vapor acting as a relaxation promoter for methane. As the laboratory was closed without people inside for the entire duration of the measurement, the CH
4 concentration can be assumed to be constant with Gaussian-distributed fluctuations. At atmospheric concentrations level, the CH
4 QEPAS signal varies linearly with absolute humidity, as demonstrated in previous studies [
25]. Thus, the CH
4 QEPAS signal is plotted as a function of the absolute humidity in
Figure 7 (black squares).
A moving average was implemented on the dataset with subset size of 25 (green circles). The corresponding calibration curve (red line) is the linear fit to averaged dataset, resulting in a slope of
m1 = 0.4 μV/ppm, an intercept of
q1 = 1.95 mV, and a R
2 of 0.98. This calibration curve was used to compensate the influence of the H
2O concentration in air on the CH
4 signal using the following equation:
where
is the signal obtained with water compensation,
is the measured CH
4 QEPAS signal, and
is the absolute humidity measured with the hygrometer.
Figure 8 shows the comparison between the CH
4 concentration detected by the QEPAS sensor without (
Figure 8a) and with (
Figure 8b) water compensation.
In both cases, the calibration curve of CH
4 in N
2 (
y = 1.07 mV/ppm
x) was used to convert the
y-axis from mV to ppm. It is worth noting that a mean value of CH
4 concentration of 6.82 ppm with a standard deviation (1σ) of 0.44 ppm was estimated without water compensation (
Figure 8a), which does not represent a reliable measurement of the atmospheric CH
4 concentration. Conversely, the use of the electronic humidity sensor allowed correct calibration of the CH
4 signal, resulting in a mean concentration value of 1.95 ± 0.25 ppm, significantly lower than the concentration estimated without water compensation and a noise comparable with that of the CH
4 sensor calibrated using the CH
4–N
2 mixtures. For comparison, the H
2O QEPAS signal was used for the correction of CH
4 QEPAS measurement instead of TSP01. The results are shown in
Figure 9.
The corrected CH
4 signal showed a trend similar to those obtained when TSP01 was used for CH
4 signal compensation (see
Figure 8b). A standard deviation of 0.27 ppm was measured, comparable with the 0.25 ppm value obtained using TSP01. As the use of the H
2O QEPAS signal as methane signal compensation does not add any improvement, we have demonstrated that the use of the hygrometer is a valid alternative when the laser spectral range does not cover a H
2O absorption line.
To verify the repeatability of the measurement, a second set of data was acquired for 48 h (the next weekend), and the same analysis was performed on this new dataset.
Figure 10 shows the CH
4 QEPAS signal (red line), the H
2O QEPAS signal (blue line), and the H
2O signal measured by the electronic hygrometer (green line) standardized to their mean and standard deviation values. Again, the influence of water on CH
4 signal is clearly visible.
As for the first dataset, the H
2O and CH
4 QEPAS signals as a function of the absolute humidity were averaged and linearly fitted; a slope of
m2 = 0.4 μV/ppm was extracted, matching the value of
m1, thus demonstrating the repeatability of the measurements. The CH
4 QEPAS signals were corrected using the linear fit with slope
m2 as calibration curve. The results without and with water calibration are shown in
Figure 11a,b, respectively, where the
y-axis has been converted in ppm using the calibration curve of CH
4 in N
2. A mean concentration value of methane in atmosphere of 1.76 ± 0.2 ppm was extracted, comparable with the previous estimation.
Although its sensitivity as well as response time is worse than H2O QEPAS detection, we have demonstrated that a hygrometer can be successfully used to compensate the CH4 QEPAS signal for a reliable detection in the atmosphere. Therefore, this approach represents a valid solution that can be easily extended for detection of all gas species with the QEPAS technique without the use of an additional laser source to target water vapor.